Abstract
Purpose
To evaluate the maximum tolerated dose (MTD) and pharmacokinetic profile of a chronomodulated, dose-intensified regimen of capecitabine in combination with oxaliplatin (XELOX) in metastatic colorectal cancer (mCRC).
Methods
Patients (N = 18) with 0 or 1 line of prior chemotherapy received oxaliplatin 100 mg/m2 on day 1 from 1400 to 1800 hours with escalating dose levels of capecitabine (2,500, 3,000, 3,500, 4,000, 4,500, and 5,000 mg) once daily taken at 2400 hours on days 1–5. Each cycle lasted 14 days.
Results
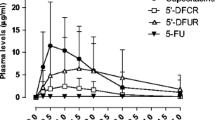
The MTD of capecitabine was 4,500 mg. Transaminitis and anemia were the commonest non-hematologic and hematologic toxicities, respectively. Toxicities were generally mild, with only five occurrences of grade 3 toxicity and none of grade 4. There were no dose-limiting toxicities, defined as specific grade 3 or 4 toxicities occurring in the first two cycles of treatment. The objective response rate was 33.3 %, and median overall survival was 16.3 months (95 % CI: 11.2–18.2 months). The maximum plasma concentration (C max) and area under plasma concentration–time curve from time 0 to infinity (AUC[0–∞]) of the capecitabine metabolites in our fixed-dosing chronomodulated regimen were comparable to values seen with comparably dose-intense regimens but associated with significantly reduced toxicity.
Conclusions
Chronomodulated dose-intensified XELOX facilitates delivery of dose-intense treatment in mCRC with a favorable therapeutic index that is promising.


Similar content being viewed by others
References
Poon MA, O’Connell MJ, Wieand HS et al (1991) Biochemical modulation of fluorouracil with leucovorin: confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol 9(11):1967–1972
Borner MM, Castiglione M, Bacchi M et al (1998) The impact of adding low-dose leucovorin to monthly 5-fluorouracil in advanced colorectal carcinoma: results of a phase III trial. Ann Oncol 9(5):535–541
de Gramont A, Bosset JF, Milan C et al (1997) Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15(2):808–815
Meta-analysis Group In Cancer (1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16(1):301–308
Lévi FA, Zidani R, Vannetzel JM et al (1994) Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst 86(21):1608–1617
Lévi F, Zidani R, Misset JL (1997) Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350(9079):681–686
Lévi F, Zidani R, Brienza S et al (1999) A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin as initial treatment of patients with metastatic colorectal carcinoma. International Organization for Cancer Chronotherapy. Cancer 85(12):2532–2540
de Gramont A, Vignoud J, Tournigand C et al (1997) Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 33(2):214–219
Giacchetti S, Bjarnason G, Garufi C et al (2006) Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol 24(22):3562–3569
Miwa M, Ura M, Nishida M et al (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumors by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34(8):1274–1281
Cassidy J, Clarke S, Díaz-Rubio E et al (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26(12):2006–2012
Rothenberg ML, Cox JV, Butts C et al (2008) Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol 19(10):1720–1726
O’quigley J, Pepe M, Fisher L (1990) Continual reassessment method: a practical design for phase I clinical trials in cancer. Biometrics 46:33–48
Goodman SN, Zahurak ML, Piantadosi S (1995) Some practical improvements in the continual reassessment method for phase I studies. Stat Med 14:1149–1161
Tan SB, Tan SH, Machin D (2005) Early phase clinical trials (EPCT) software. UKCCSG Data Centre, Leicester
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Zufía L, Aldaz A, Giráldez J (2004) Simple determination of capecitabine and its metabolites by liquid chromatography with ultraviolet detection in a single injection. J Chromatogr B Analyt Technol Biomed Life Sci 809(1):51–58
Lévi F, Okyar A, Dulong S et al (2010) Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol 50:377–421
Levi F, Schibler U (2007) Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 47:593–628
Smaaland R, Laerum OD, Sothern RB et al (1992) Colony-forming unit-granulocyte-macrophage and DNA synthesis of human bone marrow are circadian stage-dependent and show covariation. Blood 79(9):2281–2287
Lévi F, Focan C, Karaboué A et al (2007) Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv Drug Deliv Rev 59(9–10):1015–1035
Scheithauer W, Kornek GV, Raderer M et al (2002) Intermittent weekly high-dose capecitabine in combination with oxaliplatin: a phase I/II study in first-line treatment of patients with advanced colorectal cancer. Ann Oncol 13(10):1583–1589
Díaz-Rubio E, Evans TR, Tabemero J et al (2002) Capecitabine (Xeloda) in combination with oxaliplatin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumors. Ann Oncol 13(4):558–565
Scheithauer W, Kornek GV, Raderer M et al (2003) Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 21(7):1307–1312
Sharma R, Rivory L, Beale P et al (2006) A phase II study of fixed-dose capecitabine and assessment of predictors of toxicity in patients with advanced/metastatic colorectal cancer. Br J Cancer 94(7):964–968
Reigner B, Blesch K, Weidekamm E (2001) Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet 40(2):85–104
Mackean M, Planting A, Twelves C et al (1998) Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol 16(9):2977–2985
Xeloda Prescribing Information [Drugs@FDA] (2011) Available from web. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020896s026lbl.pdf. Accessed 28 Apr 2011
Mulkerin D, LoConte NK, Holen KD et al (2009) A phase I study of an oral simulated FOLFOX with high dose capecitabine. Invest New Drugs 27(5):461–468
Santini D, Vincenzi B, Schiavon G et al (2007) Chronomodulated administration of oxaliplatin plus capecitabine (XELOX) as first line chemotherapy in advanced colorectal cancer patients: phase II study. Cancer Chemother Pharmacol 59(5):613–620
Tournigand C, André T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22(2):229–237
Acknowledgments
Authors are thankful to the National Medical Research Council grant NMRC/0994/2005 for supporting the study and the patients who participated in the study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farid, M., Chowbay, B., Chen, X. et al. Phase I pharmacokinetic study of chronomodulated dose-intensified combination of capecitabine and oxaliplatin (XELOX) in metastatic colorectal cancer. Cancer Chemother Pharmacol 70, 141–150 (2012). https://doi.org/10.1007/s00280-012-1895-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1895-x