Abstract
Background
The combination of 5-fluorouracil (5-FU), leucovorin (LV), and oxaliplatin (I-OHP) was shown to be both more active against metastatic colorectal carcinoma and better tolerated if the drug delivery rate was chronomodulated according to circadian rhythms rather than constant. The aim of the present study was to define the feasibility and efficacy of XELOX administered through a new chronomodulated schedule in untreated advanced colorectal cancer (CRC) patients.
Methods
Chemotherapy-naive patients with advanced CRC were considered eligible for the study accrual. Treatment: oxaliplatin 70 mg/m2 continuous infusion (c.i.) for 12 h (8:00 a.m. to 8:00 p.m.) days 1, 8 plus chronomodulated oral capecitabine 1,750 mg/m2/die (h 8:00 a.m. 25% of total dose; h 6:00 p.m. 25% of total dose; h 11:00 p.m. 50% of total dose), days 1–14 every 21 days.
Results
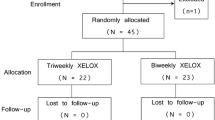
Forty-six patients were evaluated for safety and efficacy (male/female, 20/26). Median age was 64 years (range 28–77 years). Median Eastern Cooperative Oncology Group performance status (PS) was 0 (range 0–1). A total of 324 cycles have been administered: median per patient 6 (range 3–10 courses). Median number of metastatic sites was 1. Metastatic sites distribution was as follows: liver (65.2%), lung (34.8%), and nodes (32.6%). Median follow-up was 14 months (range 6.0–40.3 months). In an intent-to-treat efficacy analysis, objective response and stable disease were recorded in 27 (58.6%) and in 16 patients (34.9%), respectively. The median response duration was 8.0 months (95% CI; 5.03–10.96 months). The median time to progression (TTP) was 9.0 months (95% CI; 6.47–11.52 months). The overall survival (OS) was not reached, with a median value > 24 months (95% CI; 23.66–36.30 months). The grade 3 toxicities were diarrhea (8.7%), liver toxicity (13.1%), fatigue (8.7%), neurotoxicity (2.2%), neutropenia (8.7%), and thrombocytopenia (2.2%).
Conclusion
This regimen resulted of particular interest for patients with untreated metastatic CRC.


Similar content being viewed by others
References
Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001) Cancer statistics. CA Cancer J Clin 5:15–36
Jemal A, Tiwari RC, Murray T, et al (2004) Cancer statistics 2004. CA Cancer J Clin 54:8–29
Nordic gastrointestinal Tumor adjuvant therapy group (1992) Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal: a randomised trial. J Clin Oncol 10:904–911
Kohne-Wompner CH, Schmoll HJ, Harstrick A, Rustum YM (1992) Chemotherapeutic strategies in metastatic colorectal cancer: an overview of current clinical trials. Semin Oncol 19:105–125
Pinedo HM, Peters GF (1988) Fluorouracil: biochemistry and pharmacology. J Clin Oncol 6:1653–1664
Scheithauer W, Rosen H, Kornek GV, et al (1993) Randomized comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 306:752–755
De Gramont A, Bosset JF, Milan C, et al (1997) Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 2:808–815
Kohne CH, Wils J, Lorenz M, et al (2003) Randomized phase III study of high-dose fluorouracil given as a weekly 24-hour infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952. J Clin Oncol 21:3721–3728
Cunningham D, Pyrhonen S, James RD, et al (1998) Randomized trial of irinotecan plus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352:1413–1418
Douillard JY, Cunningham D, Roth AD, et al (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041–1047
Saltz LB, Cox JV, Blanke C, et al (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
Misset JL, Bleiberg H, Sutherland W, et al (2000) Oxaliplatin clinical activity: a review. Crit Rev Oncol Hematol 35:75–93
Raymond E, Faivre S, Woynarowski JM, Chaney SG (1998) Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol 25:4–12
De Gramont A, Figer A, Seymour M, et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Goldberg RM, Morton RF, Sargent DJ, et al (2003) N9741: oxaliplatin (oxal) or CPT-11 + 5-fluorouracil (5-FU)/leucovorin (LV) or oxal + CPT-11 in advanced colorectal cancer. Updated efficacy and quality of life (QOL) data from an intergroup study. Proc ASCO 22:252
Diaz-Rubio E, Evans TR, Tabemero J, et al (2002) Capecitabine (Xeloda) in combination with oxaliplatin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumours. Ann Oncol 13:558–565
Miwa M, Ura M, Nishida M, et al (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1281
Reigner B, Blesch K, Weidekamm E (2001) Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet 40:85–104
Hoff PM, Ansari R, Batist G, et al (2001) Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 19:2282–2292
Diaz-Rubio E, Evans TR, Tabernero J, et al (2002) Capecitabine (Xeloda) in combination with oxaliplatin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumours. Ann Oncol 13:558–565
Borner MM, Dietrich D, Stupp R, et al (2002) Phase II study of capecitabine and oxaliplatin in first- and second-line treatment of advanced or metastatic colorectal cancer. J Clin Oncol 20:1759–1766
Zeuli M, Nardoni C, Pino MS, et al (2003) Phase II study of capecitabine and oxaliplatin as first-line treatment in advanced colorectal cancer. Ann Oncol 14:1378–1382
Scheithauer W, Kornek GV, Raderer M, et al (2003) Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2:1307–1312
Milano G, Chamorey AL (2002) Clinical pharmacokinetics of 5-fluorouracil with consideration of chronopharmacokinetics. Chronobiol Int 19:177–189
Garufi C, Brienza S, Pugliese P, et al (2000) Overcoming resistance to chronomodulated 5-fluorouracil and folinic acid by the addition of chronomodulated oxaliplatin in advanced colorectal cancer patients. Anticancer Drugs 11:495–501
Mormont MC, Waterhouse J, Bleuzen P, et al (2000) Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res 6:3038–3045
Giacchetti S, Perpoint B, Zidani R, et al (2000) Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 18:136–147
Levi F, Zidani R, Brienza S, et al (1999) A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin as initial treatment of patients with metastatic colorectal carcinoma. International Organization for Cancer Chronotherapy. Cancer 85:2532–2540
Rosati G, Rossi A, Tucci A, et al (2001) Phase I study of a weekly schedule of oxaliplatin, high-dose leucovorin, and infusional fluorouracil in pretreated patients with advanced colorectal cancer. Ann Oncol 12:669–674
Santini D, Vincenzi B, La Cesa A, et al (2005) Continuous infusion of oxaliplatin plus chronomodulated capecitabine in 5-fluorouracil- and irinotecan-resistant advanced colorectal cancer patients. Oncology 69(1):27–34
MacDonald J, Haller D, Mayer R (1995) Grading of toxicity. In: MacDonald J, Haller D, Mayer R (eds) Manual of oncologic therapeutics. Lippincott, Philadelphia, PA, pp 519–523
Thall PF, Simon R, Ellenberg SS (1989) A two-stage design for choosing among several experimental treatments and a control in clinical trials. Biometrics 45:537–547
Cassidy J, Tabernero J, Twelves C, et al (2004) XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 22:2084–2091
Park SH, Bang SM, Cho EK, et al (2004) First-line chemotherapy with irinotecan plus capecitabine for advanced colorectal cancer. Oncology 66:353–357
Borner MM, Bernhard J, Dietrich D, et al (2005) A randomized phase II trial of capecitabine and two different schedules of irinotecan in first-line treatment of metastatic colorectal cancer: efficacy, quality-of-life and toxicity. Ann Oncol 16:282–288
Borner MM, Dietrich D, Stupp R, et al (2002) Phase II study of capecitabine and oxaliplatin in first- and second-line treatment of advanced or metastatic colorectal cancer. J Clin Oncol 20(1):1759–1766
Sumpter K, Harper-Wynne C, Cunningham D, et al (2003) Oxaliplatin and capecitabine chemotherapy for advanced colorectal cancer: a single institution’s experience. Clin Oncol (R Coll Radiol) 15:221–226
Pfeiffer P, Hahn P, Jensen HA (2003) Short-time infusion of oxaliplatin (Eloxatin) in combination with capecitabine (Xeloda) in patients with advanced colorectal cancer. Acta Oncol 42:832–836
Shields AF, Zalupski MM, Marshall JL, Meropol NJ (2004) Treatment of advanced colorectal carcinoma with oxaliplatin and capecitabine: a phase II trial. Cancer 100:531–537
Jordan K, Kellner O, Kegel T, et al (2004) Phase II trial of capecitabine/irinotecan and capecitabine/oxaliplatin in advanced gastrointestinal cancers. Clin Colorectal Cancer 4:46–50
Andrè T, Bensmaine MA, Louvet C, et al (1999) Multicentre phase II study of bimonthly high dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 17:3560–3568
Hochster H, Chachoua A, Speyer J, et al (2003) Oxaliplatin with weekly bolus fluorouracil and low-dose leucovorin as first-line therapy for patients with colorectal cancer. J Clin Oncol 21:2703–2707
Tournigand C, Andre T, Achille E, et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Sorbye H, Glimelius B, Berglund A, et al (2004) Multicenter phase II study of Nordic fluorouracil and folinic acid bolus schedule combined with oxaliplatin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 22:31–38
Simpson D, Dunn C, Curran M, Goa KL (2003) Oxaliplatin: a review of its use in combination therapy for advanced metastatic colorectal cancer. Drugs 63:2127–2156
Goldberg RM, Sargent DJ, Morton RF, et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30
Cassidy J, Twelves C, Van Cutsem E, et al (2002) First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 13:566–575
Hoff PM, Pazdur R, Lassere Y, et al (2004) Phase II study of capecitabine in patients with fluorouracil-resistant metastatic colorectal carcinoma. J Clin Oncol 22:2078–2083
Scheithauer W, McKendrick J, Begbie S, et al (2003) Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol 14:1735–1743
Van Cutsem E, Twelves C, Cassidy J, et al (2001) Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19:4097–4106
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santini, D., Vincenzi, B., Schiavon, G. et al. Chronomodulated administration of oxaliplatin plus capecitabine (XELOX) as first line chemotherapy in advanced colorectal cancer patients: phase II study. Cancer Chemother Pharmacol 59, 613–620 (2007). https://doi.org/10.1007/s00280-006-0302-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0302-x