Abstract
Purpose
The purpose of this study was to clarify the safety and efficacy of combination chemotherapy of uracil-tegafur (UFT) and doxorubicin (UFD regimen), and to identify the prognostic factors in patients with unresectable advanced biliary tract cancer who received systemic chemotherapy.
Methods
Patients with histologically or cytologically confirmed, measurable biliary tract cancer, including intrahepatic or extrahepatic cholangiocarcinoma, gallbladder cancer, and ampullary cancer, who were not suitable candidates for surgery, were eligible for the study. Patients received oral UFT at 300 mg/m2 per day divided into two doses on days 1–14 and intravenous doxorubicin at 30 mg/m2 on day 1. This cycle was repeated every 21 days. The relationship between the patient characteristics and the prognosis was examined. Univariate and multivariate analyses were conducted to identify the prognostic factors associated with survival.
Results
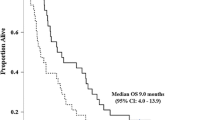
Sixty-one patients from 12 institutions were enrolled in the late phase II study between April 2005 and March 2006. Of the 61 patients, 4 patients had partial responses, for an objective response rate of 6.6% (95% CI: 1.8–15.9%); 28 patients had stable disease, 27 had progressive diseases, and 2 patients were not evaluated. The median progression-free survival was 1.6 months, and the overall median survival time was 6.5 months. In the 85 patients who received this UFD chemotherapy in previous and late phase II studies, multivariate analysis revealed the ECOG performance status 1 (P = 0.001), gallbladder as the primary cancer site (P = 0.014), T-factor 4 of the TNM classification (P = 0.035), and elevated serum lactate dehydrogenase levels (P = 0.043) as being associated with a significantly shorter survival.
Conclusions
Combination chemotherapy of UFT and doxorubicin had minimum activity against advanced biliary tract cancer. Performance status was identified as the most important prognostic factor in patients who received systemic chemotherapy.



Similar content being viewed by others
References
Sobin LH, Wittekind CH (eds) (2002) TNM classification of malignant tumours. Liver, UICC, 6th edn. Wiley-Liss, New York, pp 82–83
National Cancer Center. Cancer statistics in Japan 2007. http://www.fpcr.or.jp/publication/statistics.html. Accessed 10 March, 2008
Glimelius B, Hoffman K, Sjoden PO et al (1996) Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 7:593–600
Hejna M, Pruckmayer M, Raderer M (1998) The role of chemotherapy and radiation in the management of biliary cancer: a review of the literature. Eur J Cancer 34:977–986
Falkson G, MacIntyre JM, Moertel CG (1984) Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer 54:965–969
Patt YZ, Jones DV Jr, Hoque A, Lozano R, Markowitz A, Raijman I et al (1996) Phase II trial of intravenous fluorouracil and subcutaneous interferon alpha-2b for biliary tract cancer. J Clin Oncol 14:2311–2315
Harvey JH, Smith FP, Schein PS (1984) 5-Fluorouracil, mitomycin, and doxorubicin (FAM) in carcinoma of the biliary tract. J Clin Oncol 2:1245–1248
Ellis PA, Norman A, Hill A et al (1995) Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer 31A:1594–1598
Morizane C, Okada S, Okusaka T, Ueno H, Saisho T (2003) Phase II study of cisplatin, epirubicin, and continuous-infusion 5-fluorouracil for advanced biliary tract cancer. Oncology 64:475–476
Rao S, Cunningham D, Hawkins RE et al (2005) Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer 92:1650–1654
Penz M, Kornek GV, Raderer M et al (2001) Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol 12:183–186
Tsavaris N, Kosmas C, Gouveris P et al (2004) Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs 22:193–198
Okusaka T, Ishii H, Funakoshi A et al (2006) Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 57:647–653
Hsu C, Shen YC, Yang CH et al (2004) Weekly gemcitabine plus 24-h infusion of high-dose 5-fluorouracil/leucovorin for locally advanced or metastatic carcinoma of the biliary tract. Br J Cancer 90:1715–1719
Alberts SR, Al-Khatib H, Mahoney MR et al (2005) Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer 103:111–118
Knox JJ, Hedley D, Oza A et al (2005) Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 23:2332–2338
Cho JY, Paik YH, Chang YS et al (2005) Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer 104:2753–2758
Doval DC, Sekhon JS, Gupta SK et al (2004) A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer 90:1516–1520
Thongprasert S, Napapan S, Charoentum C et al (2005) Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol 16:279–281
Andre T, Tournigand C, Rosmorduc O et al (2004) Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 15:1339–1343
Kuhn R, Hribaschek A, Eichelmann K et al (2002) Outpatient therapy with gemcitabine and docetaxel for gallbladder, biliary, and cholangio-carcinomas. Invest New Drugs 20:351–356
McWilliams RR, Foster NR, Quevedo FJ et al (2007) NCCTG phase I/II trial (N9943) of gemcitabine and pemetrexed in patients with biliary tract or gallbladder carcinoma: phase II results. J Clin Oncol, Proc Am Soc Clin Oncol 25:217s (abstr 4578)
Fujii S, Ikenaka K, Fukushima M, Shirasaka T (1978) Effect of uracil and its derivatives on antitumor activity of 5-fluorouracil and 1-(2-tetrahydrofuryl)-5-fluorouracil. Jpn J Cancer Res (Gann) 69:763–772
Pazdur R, Lassere Y, Diaz-Canton E, Bready B, Ho DH (1996) Phase I trials of uracil-tegafur (UFT) using 5 and 28 day administration schedules: demonstration of schedule-dependent toxicities. Anticancer Drugs 7:728–733
Furuse J, Okusaka T, Funakoshi A, Yamao K, Nagase M, Ishii H et al (2006) Early phase II study of uracil-tegafur plus doxorubicin in patients with unresectable advanced biliary tract cancer. Jpn J Clin Oncol 36:552–556
Furuse J, Okusaka T, Boku N, Ohkawa S, Sawaki A, Masumoto T, Funakoshi A (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62(5):849–855
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96:896–902
Furuse J, Takada T, Miyazaki M et al (2008) Guidelines for chemotherapy of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg 15:55–62
Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, Boku N, Tanaka K, Nagase M, Saisho H, Sato T (2007) A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol 37:843–851
Acknowledgments
This study was supported in part by Grants-in-Aid for Cancer Research from the Ministry of Health, Labour, and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furuse, J., Okusaka, T., Ohkawa, S. et al. A phase II study of uracil-tegafur plus doxorubicin and prognostic factors in patients with unresectable biliary tract cancer. Cancer Chemother Pharmacol 65, 113–120 (2009). https://doi.org/10.1007/s00280-009-1011-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1011-z