Abstract
Purpose
This phase I study characterized the pharmacokinetics of free and total platinum derived from cisplatin administered alone and in combination with pemetrexed. Secondary objectives were to assess the pharmacokinetics of pemetrexed when it is combined with cisplatin as well as to evaluate the safety profile and document antitumor activity associated with this combination.
Methods
An open-label, two-arm, cross-over phase 1 study was performed in patients with squamous cell carcinoma of the head and neck, age ≥18 years, an Eastern Cooperative Oncology Group performance status of 0–2, and adequate organ function. Blood samples were taken and pharmacokinetics evaluated for the first two cycles using noncompartmental analysis. Patients received either pemetrexed (500 mg m−2) plus cisplatin (75 mg m−2) administered in cycle 1 followed by cisplatin alone in cycle 2; or in the reverse order (i.e., cisplatin alone in cycle 1 followed by pemetrexed plus cisplatin in cycle 2). Each treatment cycle was 21 days and patients received folic acid, vitamin B12 supplementation, and dexamethasone prophylaxis. After the first two cycles, patients continued study treatment with pemetrexed plus cisplatin every 3 weeks up to a maximum of six total treatment cycles. Toxicities were graded by the investigators according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE), version 3.0.
Results
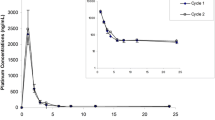
A total of 13 patients were treated; one patient was discontinued from the study after cycle 1 for failure to meet baseline eligibility criteria for renal function. The ratios and 90% confidence intervals (CI) comparing the pharmacokinetics for cisplatin administered with pemetrexed to those for cisplatin administered alone for free platinum were: C max = 1.08 (CI: 0.92, 1.27) and AUC = 0.93 (CI: 0.82, 1.06); and, total platinum were: C max = 0.97 (CI: 0.88, 1.06) and AUC = 0.87 (CI: 0.81, 0.93). These results indicate that platinum pharmacokinetics (free and total) are similar, whether cisplatin is administered alone or combined with pemetrexed. The pemetrexed pharmacokinetic results were consistent with those from previous single-agent pemetrexed studies and a previous study of pemetrexed in combination with cisplatin. The combination of pemetrexed and cisplatin did not show any unexpected toxicities. Consistent with the platinum pharmacokinetic results, co-administration with pemetrexed did not appear to enhance cisplatin-related toxicities. Of the 13 treated patients, 11 had stable disease as the best overall response and 2 had progressive disease.
Conclusions
The pharmacokinetics of free platinum derived from cisplatin were not altered by co-administration with pemetrexed, and in agreement with this, no unexpected cisplatin-induced toxicities were observed when these drugs were combined.


Similar content being viewed by others
References
Shih C, Habeck LL, Mendelsohn LG, Chen VJ, Schultz RM (1998) Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine-based antifolate LY231514 (MTA). Adv Enzyme Regul 38:135–152
Hanauske AR, Chen V, Paoletti P, Niyikiza C (2001) Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist 6:363–373
Vogelzang NJ, Rusthoven JJ, Symanowski J et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
Hanna N, Shepherd FA, Fossella FV et al (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597
Scagliotti G, Parikh P, von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol, in press
ALIMTA® (2007) Pemetrexed for injection (package insert). Eli Lilly and Company, Indianapolis, IN, USA. Available via: http://pi.lilly.com/us/alimta-pi.pdf. Accessed 08 May 2008
The NCCN Clinical Practice Guidelines in Oncology™ (2007) Head and Neck Cancers (Version 1). National Comprehensive Cancer Network, Inc. Available via: http://www.nccn.org/professionals/physician_gls/PDF/head-and-neck.pdf. Accessed 08 May 2008
Pivot X, Raymond E, Laguerre B et al (2001) Pemetrexed disodium in recurrent locally advanced or metastatic squamous cell carcinoma of the head and neck. Br J Cancer 85:649–655
Thodtmann R, Depenbrock H, Dumez H et al (1999) Clinical and pharmacokinetic phase I study of multitargeted antifolate (LY231514) in combination with cisplatin. J Clin Oncol 17:3009–3016
Eli Lilly and Company (2006) A study for patients with head and neck cancer [H3E-MC-JMHR]. ClinicalTrials.gov. Available via: http://www.clinicaltrials.gov/ct2/show/NCT00415194?term=H3E-MC-JMHR&rank=1. Accessed 08 May 2008
PLATINOL®-AQ (1999) Cisplatin for injection (package insert). Bristol-Myers Squibb Company, Princeton, NJ, USA. Available via: http://165.112.6.82/dailymed/fda/fdaDrugXsl.cfm?id=4915&type=display. Accessed 08 May 2008
Cancer Therapy Evaluation Program (2006) Common terminology criteria for adverse events v3.0 (CTCAE). National Cancer Institute. Available via: http://ctep.cancer.gov/forms/CTCAEv3.pdf. Accessed 08 May 2008
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Vermorken JB, van der Vijgh WJ, Klein I et al (1984) Pharmacokinetics of free and total platinum species after short-term infusion of cisplatin. Cancer Treat Rep 68:505–513
Vermorken JB, van der Vijgh WJ, Klein I et al (1986) Pharmacokinetics of free and total platinum species after rapid and prolonged infusions of cisplatin. Clin Pharmacol Ther 39:136–144
Latz JE, Chaudhary A, Ghosh A, Johnson RD (2006) Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol 57:401–411
Musib LC, Schneck KB, Thomas M et al (2008) Pharmacokinetics of platinum (total and free) and pemetrexed for pemetrexed doses of 500 mg/m2 to 900 mg/m2 administered in combination with cisplatin 75 mg/m2. ICACT, abstract PO 215. Available via: http://www.icact.com/Abstract_Book_2008.pdf. Accessed 08 May 2008
Takimoto CH, Hammond-Thelin LA, Latz JE et al (2007) Phase I and pharmacokinetic study of pemetrexed with high-dose folic acid supplementation or multivitamin supplementation in patients with locally advanced or metastatic cancer. Clin Cancer Res 13:2675–2683
Niyikiza C, Baker SD, Seitz DE et al (2002) Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther 1:545–552
Rinaldi DA, Kuhn JG, Burris HA et al (1999) A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol 44:372–380
Acknowledgments
The authors thank Stacey E. Shehin, PhD, for assistance in preparation of this manuscript, Joke Dyck and Katleen Aelbrecht for their efforts in collecting and processing the blood samples required for pharmacokinetic analysis, and the patients for their willingness to participate in the study. This study was sponsored and funded by Eli Lilly and Company, Indianapolis, IN, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Specenier, P.M., Ciuleanu, T., Latz, J.E. et al. Pharmacokinetic evaluation of platinum derived from cisplatin administered alone and with pemetrexed in head and neck cancer patients. Cancer Chemother Pharmacol 64, 233–241 (2009). https://doi.org/10.1007/s00280-008-0853-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0853-0