Abstract
Purpose
This phase I study endeavored to estimate the maximum tolerated dose and describe the dose-limiting toxicities (DLTs) of oral irinotecan with gefitinib in children with refractory solid tumors.
Methods
Oral irinotecan was administered on days 1–5 and 8–12 with oral gefitinib (fixed dose, 150 mg/m2/day) on days 1–12 of a 21-day course. The escalation with overdose control method guided irinotecan dose escalation (7 dose levels, range 5–40 mg/m2/day).
Results
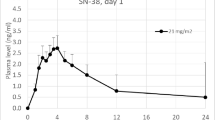
Sixteen of 19 patients were evaluable, with serial pharmacokinetic studies in ten patients. Diagnoses included osteosarcoma (N = 5), neuroblastoma (N = 3), sarcoma (N = 3), and others (N = 5). Patients received a median of two courses (range 1–20), with at least two patients treated on dose levels 2–7. Three patients had five DLTs; the most common being metabolic (hypokalemia, N = 2 and hypophosphatemia, N = 1) at dose levels two (10 mg/m2) and four (20 mg/m2). One patient experienced grade 3 diarrhea (40 mg/m2). Irinotecan bioavailability was 2.5-fold higher when co-administered with gefitinib, while the conversion rate of irinotecan to SN-38 lactone was unaffected. The study closed due to poor accrual before evaluation of the next recommended irinotecan dose level (35 mg/m2). Of 11 patients receiving at least two courses of therapy, three had stable disease lasting two to four courses and one patient maintained a complete response through 18 courses.
Conclusions
The combination of oral gefitinib and irinotecan has acceptable toxicity and anti-tumor activity in pediatric patients with refractory solid tumors. Pharmacokinetic analysis confirms that co-administration of gefitinib increases irinotecan bioavailability leading to an increased SN-38 lactone systemic exposure.


Similar content being viewed by others
References
Tsuji T, Kaneda N, Kado K et al (1991) CPT-11 converting enzyme from rat serum: purification and some properties. J Pharmacobiodyn 14:341–349
Bomgaars L, Kerr J, Berg S et al (2006) A phase I study of irinotecan administered on a weekly schedule in pediatric patients. Pediatr Blood Cancer 46:50–55
Bomgaars LR, Bernstein M, Krailo M et al (2007) Phase II trial of irinotecan in children with refractory solid tumors: a children’s oncology group study. J Clin Oncol 25:4622–4627
Dharmarajan KV, Wexler LH, Wolden SL (2013) Concurrent radiation with irinotecan and carboplatin in intermediate- and high-risk rhabdomyosarcoma: a report on toxicity and efficacy from a prospective pilot phase II study. Pediatr Blood Cancer 60:242–247
Mascarenhas L, Lyden ER, Breitfeld PP et al (2010) Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the children’s oncology group. J Clin Oncol 28:4658–4663
Mixon BA, Eckrich MJ, Lowas S, Engel ME (2013) Vincristine, irinotecan, and temozolomide for treatment of relapsed alveolar rhabdomyosarcoma. J Pediatr Hematol Oncol 35:e163–e166
Rodriguez-Galindo C, Crews KR, Stewart CF et al (2006) Phase I study of the combination of topotecan and irinotecan in children with refractory solid tumors. Cancer Chemother Pharmacol 57:15–24
Shitara T, Shimada A, Hanada R et al (2006) Irinotecan for children with relapsed solid tumors. Pediatr Hematol Oncol 23:103–110
Vassal G, Couanet D, Stockdale E et al (2007) Phase II trial of irinotecan in children with relapsed or refractory rhabdomyosarcoma: a joint study of the French Society of Pediatric Oncology and the United Kingdom Children’s Cancer Study Group. J Clin Oncol 25:356–361
Vassal G, Doz F, Frappaz D et al (2003) A phase I study of irinotecan as a 3-week schedule in children with refractory or recurrent solid tumors. J Clin Oncol 21:3844–3852
Blaney S, Berg SL, Pratt C et al (2001) A phase I study of irinotecan in pediatric patients: a pediatric oncology group study. Clin Cancer Res 7:32–37
Cosetti M, Wexler LH, Calleja E et al (2002) Irinotecan for pediatric solid tumors: the Memorial Sloan-Kettering experience. J Pediatr Hematol Oncol 24:101–105
Furman WL, Stewart CF, Poquette CA et al (1999) Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol 17:1815–1824
Mugishima H, Matsunaga T, Yagi K et al (2002) Phase I study of irinotecan in pediatric patients with malignant solid tumors. J Pediatr Hematol Oncol 24:94–100
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Wu YL, Chu DT, Han B et al (2012) Phase III, randomized, open-label, first-line study in Asia of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer: evaluation of patients recruited from mainland China. Asia Pac J Clin Oncol 8:232–243
Gao Z, Han B, Wang H et al (2012) Clinical observation of gefitinib as a first-line therapy in sixty-eight patients with advanced NSCLC. Oncol Lett 3:1064–1068
Zhang L, Ma S, Song X et al (2012) Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 13:466–475
Houghton PJ, Cheshire PJ, Harwood FG (2000) Evaluation of ZD1839 (gefitinib) alone and in combination with irinotecan (CPT-11) against pediatric solid tumor xenografts. In: Proceedings of the 11th NCI-EORTC-AACR symposium, Amsterdam, The Netherlands, November 7–10, 2000. Clin Cancer Res 2000 (Suppl 6):379
Daw NC, Furman WL, Stewart CF et al (2005) Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: a children’s oncology group study. J Clin Oncol 23:6172–6180
Furman WL, Navid F, Daw NC et al (2009) Tyrosine kinase inhibitor enhances the bioavailability of oral irinotecan in pediatric patients with refractory solid tumors. J Clin Oncol 27:4599–4604
Babb J, Rogatko A, Zacks S (1998) Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med 17:1103–1120
Owens TS, Dodds H, Fricke K et al (2003) High-performance liquid chromatographic assay with fluorescence detection for the simultaneous measurement of carboxylate and lactone forms of irinotecan and three metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 788:65–74
Furman WL, Crews KR, Billups C et al (2006) Cefixime allows greater dose escalation of oral irinotecan: a phase I study in pediatric patients with refractory solid tumors. J Clin Oncol 24:563–570
Crews KR, Stewart CF, Jones-Wallace D et al (2002) Altered irinotecan pharmacokinetics in pediatric high-grade glioma patients receiving enzyme-inducing anticonvulsant therapy. Clin Cancer Res 8:2202–2209
Ma MK, Zamboni WC, Radomski KM et al (2000) Pharmacokinetics of irinotecan and its metabolites SN-38 and APC in children with recurrent solid tumors after protracted low-dose irinotecan. Clin Cancer Res 6:813–819
Thompson PA, Gupta M, Rosner GL et al (2008) Pharmacokinetics of irinotecan and its metabolites in pediatric cancer patients: a report from the children’s oncology group. Cancer Chemother Pharmacol 62:1027–1037
Stewart CF, Leggas M, Schuetz JD et al (2004) Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res 64:7491–7499
Wagner LM, Crews KR, Iacono LC et al (2004) Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clin Cancer Res 10:840–848
Caldwell PH, Murphy SB, Butow PN, Craig JC (2004) Clinical trials in children. Lancet 364:803–811
Skolnik JM, Barrett JS, Jayaraman B et al (2008) Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol 26:190–195
Le Tourneau C, Lee JJ, Siu LL (2009) Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 101:708–720
Lee DP, Skolnik JM, Adamson PC (2005) Pediatric phase I trials in oncology: an analysis of study conduct efficiency. J Clin Oncol 23:8431–8441
Gururangan S, Fangusaro J, Poussaint TY et al (2014) Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—a pediatric brain tumor consortium study. Neuro Oncol 16:310–317
Wagner LM, McAllister N, Goldsby RE et al (2007) Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer 48:132–139
Casey DA, Wexler LH, Merchant MS et al (2009) Irinotecan and temozolomide for Ewing sarcoma: the memorial sloan-kettering experience. Pediatr Blood Cancer 53:1029–1034
Zhang YT, Feng LH, Zhong XD et al. Vincristine and irinotecan in children with relapsed hepatoblastoma: A single-institution experience. pediatr hematol oncol 2014
Qayed M, Powell C, Morgan ER et al (2010) Irinotecan as maintenance therapy in high-risk hepatoblastoma. Pediatr Blood Cancer 54:761–763
Trobaugh-Lotrario AD, Katzenstein HM (2012) Chemotherapeutic approaches for newly diagnosed hepatoblastoma: past, present, and future strategies. Pediatr Blood Cancer 59:809–812
Acknowledgments
This work was supported in part by a grant from AstraZeneca (Protocol 1839US/0300), which provided partial funding as well as study drug for participants. The manuscript was reviewed and approved prior to publication. We thank the American Lebanese Syrian Associated Charities for their support, Amy Sanders and Dana Hawkins for facilitating data collection, Dr. Barry Shulkin for providing diagnostic images and Dr. Alberto Pappo for editorial assistance.
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brennan, R.C., Furman, W., Mao, S. et al. Phase I dose escalation and pharmacokinetic study of oral gefitinib and irinotecan in children with refractory solid tumors. Cancer Chemother Pharmacol 74, 1191–1198 (2014). https://doi.org/10.1007/s00280-014-2593-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2593-7