Abstract
Purpose
The current study assessed the efficacy and safety of biweekly oxaliplatin combining oral tegafur–uracil/leucovorin in treating chemonaive patients with advanced gastric cancer.
Methods
Eligible patients were 18–75 years old, had stage IV disease or post-surgery recurrence, no prior palliative chemotherapy, and an ECOG performance status of 0–2. Patients in the current study received 2-h i.v. infusion of oxaliplatin at a dose of 100 mg/m2 after diluting in 500 mL 5% dextrose/water (dexan premedication), and 5-HT3 antagonist biweekly. Oral tegafur–uracil and leucovorin was given at a dose of 300 mg/m2/day and 60 mg/day three times daily from day 1 to 21, respectively, followed by a 1-week rest. Response assessment was based on the RECIST criteria and was performed every two courses. Toxicity was assessed according to NCI common toxicity criteria version 2.
Results
From October 2003 to April 2006, 57 patients were evaluated (55 eligible) with a median age of 61 years (range 31–75). According to the assessment of response in 48 evaluable patients, partial response rate was 24/48 (50.0%) (95% CI: 35.23–64.73%) and stable disease was observed in 11 patients (22.92%), and diseased progressed in 13 patients (27.08%). Mean number of oxaliplatin cycles was 3 (0.5–6.5). Median time to progression was 177 days. Median overall survival was 318 days. Major-grade (III/IV) toxicities were diarrhea 25.5%, vomiting 16.5%, anemia 10.9%, numbness 12.7%, thrombocytopenia 7.3%, neutropenia 3.6% and leucopenia 1.8%.
Conclusions
Biweekly, oxaliplatin combining oral tegafur–uracil/leucovorin in treating patients with advanced gastric cancer showed acceptable activity and manageable toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric adenocarcinoma is a common cancer and common cause of cancer deaths worldwide [1]. Most patients receive curative surgical resection; however, many patients eventually relapse with local-regional recurrence or distant metastases [2]. Approximately 20–30% of patients have inoperable disease at diagnosis. Thus, the majority of patients require palliative treatment at certain time-points. Randomized trials have demonstrated that 5-fluorouracil-based (5-FU) or cisplatin-based (CDDP) chemotherapeutic regimens may improve survival and quality of life in patients with advanced gastric cancer compared with best supportive care [3, 4]. Although there are no standard combination regimens for advanced gastric cancer, 5-FU-based and CPPD-based regimens are mostly used in clinical practice [4, 5].
Tegafur–uracil (uracil combined with tegafur in a 4:1 ratio) is a second-generation, oral 5-FU pro-drug. Uracil prevents the degradation of 5-FU by inhibiting dehydropyrimidine dihydrogenase (DPD), and this leads to increased concentrations of 5-FU in plasma and tumor tissue [6]. Tegafur–uracil, which is widely used in Asia, has been approved for use in the treatment of metastatic colorectal, gastric and breast cancer in our country, Taiwan. Prolonged use of tegafur–uracil results in a similar or higher maximum concentration (C max) and area under the curve (AUC) compared to that achieved by giving continuous infusion of 5-FU; however, pharmacokinetic patterns differ [7, 8]. Phase II data suggested that tegafur–uracil and 5-FU had similar activity against gastric cancer [9]. Patients’ tolerance to tegafur–uracil on a schedule of 28 days on/7 days off has been excellent. Common toxicities of tegafur–uracil include anorexia, nausea and vomiting; diarrhea and hematologic toxicity is rare. We hypothesized that it was worthwhile to study tegafur–uracil combining with other active and novel chemotherapeutic agents to increase the response rate and improve the survival of patients with advanced gastric cancer.
Oxaliplatin (OXA), a new cytotoxic agent from the diaminocyclohexane platinum family, has a mechanism of action similar to that of other platinum derivatives, but its spectrum of antitumor activity against tumor models differs from those of CDDP and carboplatin [10]. Experiments have shown activity against CDDP-resistant colon carcinoma cell lines and the synergistic activity of combined OXA and 5-FU [10]. OXA’s clinical toxicity is distinct from that of other platinum drugs in terms of the absence of renal toxicity and minimal incidence of hematotoxicity; it causes both reversible acute, cold-related dysesthesia and dose-limiting cumulative peripheral sensory neuropathy [11]. Its activity as a single agent in either chemonaive or 5-FU pretreated patients with metastatic colorectal cancer has been demonstrated in phase II trials; response rates ranged from 10 to 24% [12, 13]. Phase III study has confirmed the role of OXA combining with 5-FU/LV as standard care, first-line therapy for treating metastatic colorectal cancer [14]. In a pilot study, patients with advanced scirrhous-type gastric cancer who were given preoperative OXA showed a clinical response with significant platinum concentration observed in tissue examined postoperatively [15]. The combination of OXA, oral tegafur–uracil and LV has shown synergistic antitumor activity against human colorectal HT29 cell xenografts in athymic nude mice [16]. To date, few data have been collected from phase I and II clinical studies investigating the use of OXA combining oral tegafur–uracil and LV in patients with gastric cancer. The advantage of such combination is that OXA does not require hydration, and tegafur–uracil/LV can be given orally in an outpatient setting. In a previous, dose-escalation study, we established the recommended doses of OXA and tegafur–uracil/LV in patients with advanced gastric cancer [17]. In the current phase II study, primary end point was the assessment of response rate. Secondary endpoints were the assessment of time to progression, median survival, and drug-related toxicity. Herein, we reported the final results of this phase II study.
Patients and methods
Eligibility criteria
Eligibility criteria were as follows. Patients had to have a histological or cytological diagnosis of gastric adenocarcinoma with metastatic disease. Patients had to be ≥18 and ≤75 years of age and have an ECOG performance status score ≤2. Patients had to have recovered from recent surgery (procedure at least 2 weeks prior to enrollment) or radiotherapy (completed at least 4 weeks prior to enrollment) and have not had any palliative chemotherapy. Patients with previous adjuvant and/or neoadjuvant chemotherapy were eligible if they had not been treated for over 6 months between the end of adjuvant chemotherapy and the first relapse. Patients had to have at least one measurable lesion at enrollment. Measurable lesions were defined as lesions that could be measured in at least one dimension as ≥20 mm with conventional technique or ≥10 mm with spiral computed tomography (CT). Previously irradiated lesions were not considered measurable target lesions. Patients were allowed to complete their initial work-up within 2 weeks prior to receiving the first cycle of chemotherapy in this trial.
Patients had to show baseline eligibility in laboratory tests as follows: neutrophils ≥ 1,500/L, platelets ≥ 100,000/L, serum creatinine ≤ 1.5 × upper limit of normal (ULN), total bilirubin ≤ 1.5 × ULN, SGOT and SGPT ≤ 2.5 × ULN (≤5.0 × ULN if hepatic metastasis present). Finally, patients had to complete an informed consent form before entering the study. This study was approved prior to implementation by the Scientific and Research Ethics Committees of the participating institutions.
Exclusion criteria
Patients with the following conditions were excluded: life expectancy less than 3 months, had central nervous system metastasis, had bone metastasis only, pregnancy or breast-feeding, clinically detectable peripheral neuropathy more than grade 2 due to any causes, concomitant illness that might be aggravated by chemotherapy, active cardiac disease within 6 months preceding entry into the study (e.g., angina or myocardial disease), history of other malignancy except for curatively treated nonmelanoma skin cancer or cervical carcinoma in situ, mental status unfit for clinical trials, hypersensitive to any components of this chemotherapeutic regimen, or intestinal obstruction, malabsorption or any other condition that precluded taking oral study medication.
Treatment schedule
Oxaliplatin (OXALIP®, TTY Biopharm Co. Ltd, Taipei, Taiwan) 100 mg/m2 was administered on days 1 and 15 as a 2-h infusion. Tegafur–uracil (UFUR® TTY Biopharm Co. Ltd, Taipei, Taiwan)/LV was orally administered from day 1 to day 21 followed by a 7-day break. The dose of tegafur–uracil was 300 mg/m2/day (based on tegafur dose) given orally in three divided doses every 8 h (approximately 7 am, 3 pm and 11 pm). If the capsule dose could not be divided equally, the highest dose was administered in the morning and the lowest dose administered in the evening. LV was supplied as 15-mg tablets and was administered orally at a dose of 60 mg/day. LV was given to the patients concurrently with tegafur–uracil; the first LV dose (7 am) was 30 mg followed by two subsequent doses of 15 mg given at 3 pm and 11 pm, respectively. Patients were instructed to take 120–240 cc water with medication and not to consume any food for 1 h before and after the ingestion of medication. The administration of tegafur–uracil/LV was to be continued if the discontinuation of OXA was required with the absence of disease progression. This regimen was repeated every 28 days as one treatment course. Treatment was continued until objective evidence of disease progression was obtained, protocol noncompliance was established or an individual patient wished to withdraw from the study at his/her own request or if an investigator felt that patient withdrawal was in the patient’s best interest (for example, if surgery became acceptable).
Study evaluation
Tumor response was evaluated after every two courses of chemotherapy according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines [18]. Toxicities were recorded based on the National Cancer Institute Common Toxicity Criteria (NCI-CTC, version 2, 1998).
Statistical considerations
The primary endpoint was response rate. Secondary endpoints were time to progression, overall survival time and drug-related toxicity. Time to progression was defined as the period from the first day of drug treatment to the date when progressive disease or relapse was clearly documented. Overall survival represented the number of days until death from any cause. Safety variables included toxicity grading, adverse events and laboratory values. Based on previous studied reports, a response rate of 30–60% with an expected mean of approximately 50% was assumed for the study regimen. Simon’s two-stage optimal design was adapted to calculate the sample size for type-I error of 0.05 and power of 80%. Based on the null-hypothesized proportion of 30% and the research-hypothesized proportion of 50%, Simon’s two-stage optimal design determined that evaluable patients for stages I and II were as follows: of the first 15 patients enrolled, >5 (or ≥6) patients were required to show treatment response to go to the second stage; otherwise, the trial would be terminated. If the response outcome passed the first stage, additional 31 patients would be recruited and >18 (or ≥19) patients were required to show a response to conclude that the study regimen was effective. The sample size was determined to yield 46 evaluable patients in the phase II trial.
Results
Patient characteristics
A total of 57 patients were screened according to study criteria from October 2003 to April 2006 at six sites. Two patients were not eligible for this study. Of the 55 eligible patients, there were 20 women and 35 men (median age 61 years; range 31–75). Patient characteristics were as shown in Table 1. Mean number of cycles of OXA administered was 3.82 (0.5–7.0). Mean cumulative dose of OXA was 1,070.67 mg (150.0–2,312.0). Mean drug compliances for tegafur–uracil and LV were 96.89% (29.0–100.0) and 97.05% (35.0–100.0), respectively.
Efficacy
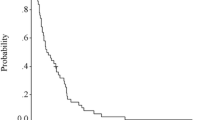
By the end of June 2007, all participants had finished receiving study treatment. The median follow-up duration was 343 days (range 16–1,239). Seven patients were not evaluated for response: one died during the first month of treatment secondary to pneumonia and gastrointestinal bleeding; four patients could not tolerate therapy; one patient had poor compliance; and one patient was removed from the study at an investigator’s discretion. The remaining 48 patients were evaluated for response: 24 patients had partial response (50.0%, 95% CI: 35.23–64.73%), 11 patients had stable disease (22.92%) and 13 patients had progression of disease (27.08%). The median time to progression and overall median survival time for all 55 patients were 177 days (95% CI: 148–224 days) and 331 days (95% CI: 253–415 days), respectively. The estimated 1-, 2- and 3-year survival rates were 41, 13 and 8%, respectively. The most common causes for discontinuation from study treatment in 48 evaluated patients were disease progression (26 patients, 54%) and adverse events (22 patients, 45.8%). The most common adverse event that led to discontinuation of participation in the study was numbness (14 patients, 29%).
Toxicity
Toxicity was assessed in all 55 patients (Table 2). The most common hematological toxicity was thrombocytopenia, and the most common nonhematological toxicity was diarrhea. The most common grade III/IV toxicity was diarrhea (14/55, 25.4%), followed by vomiting (9/55, 16.4%), numbness (12/55, 12.7%), anemia (6/55, 10.9%), nausea (5/55, 9.1%), thrombocytopenia (4/55, 7.3%) and neutropenia (3/55, 5.5%).
Discussion
Since 2002, biweekly or weekly, OXA with 5FU-LV given as 24-h or 48-h infusion by pump has been studied in several phase II trials involving patients with advanced gastric cancer [19–22]. Reported response rates have ranged from 43 to 56%, with time to progression ranging from 5.2 to 6.5 months and overall median survival ranging from 8.6 to 11.4 months, respectively. Major toxicities were myelotoxicity and neuropathy. In comparison with previous regimens, OXA and 5-FU/LV combination showed better efficacy and tolerance. Therefore, authors encouraged the initiation of further phase III studies involving such regimen.
Oral tegafur–uracil/LV is commonly used for gastric cancer in Asia and appears to have similar efficacy with bolus 5-FU/LV in patients with advanced colorectal cancer. OXA combining with oral tegafur–uracil/LV without using pumps appears to be an attractive alternative to infusion of 5-FU/LV. Such combination chemotherapy treatment used in the current study produced a 50% response rate, time to progression of 177 days and median survival of 331 days. The major toxicity was diarrhea. Results of this phase II study resemble those of the previous phase I dose-finding study [17]. The major barrier for the patients to receive complete doses of this regimen was the occurrence of diarrhea. The results were comparable with those of several previous phase II studies using the combination OXA and infusion 5-FU/LV [19–22]. Efficacy and median survival were similar to the results shown in previous reports with the exception of toxicity profiles. Grade III/IV myelotoxicity in the current study was less than 10%. In contrast, the regimen of OXA combining infusional 5-FU/LV induced a much higher rate of myelotoxicity (more than 20%) and less frequent rate of diarrhea (less than 10%). Jatoi et al. reported that OXA and oral capcetabine for advanced gastroesophageal cancer was associated with grade III/IV diarrhea in 30% of patients [23]. Park et al. used the same schedule as Jatoi et al.’s study for 20 patients with advanced gastric cancer [24]. The response rate reported in their study was 65%, with grade III/IV toxicities of diarrhea, neutropenia and vomiting (all in less than 5% of patients). Obviously, the major limitation for clinical application of OXA and oral tegafur–uracil/LV is diarrhea instead of hand-foot syndrome. Our suggestions for preventing severe diarrhea are as follows: firstly, reduce OXA dose to 85 mg/m2 biweekly in future trials based on experience from our initial dose-escalation study [17]; secondly, avoid the use of this combination in patients who present with poor baseline gastrointestinal function; thirdly, replace tegafur–uracil/LV with S-1 in future trials. S-1 is a novel oral fluroropyrimidine, which was reported to have a single agent response rate of 44% in treating advanced gastric cancer with a grade III/IV diarrhea rate of only 2% [25].
Efficacy and toxicity of OXA and oral tegafur–uracil/LV have not yet been reported in the treatment of gastric cancer, but both chemotherapeutic agents have been studied extensively in colorectal cancer in both advance and adjuvant setting. The Oncopaz Cooperative Group used OXA 85 mg/m2 biweekly with oral tegafur–uracil 390 mg/m2/day and oral LV in treating patients with advanced colon cancer [26]. In the beginning of the study, the first 16 patients showed 56% incidence rate of grade III/IV diarrhea; after the dose of tegafur–uracil was reduced to 300 mg/m2/day for the next 66 patients, the incidence rate of grade III/IV diarrhea was reduced dramatically to 21%. In another study, which enrolled patients older than 70 years who had advanced colorectal cancer, OXA was given at a dose of 65 mg/m2 on days 1 and 8 with oral tegafur–uracil 300 mg/m2/day and LV 90 mg/day given for 14 days in a 3-week schedule [27]. The authors concluded that this combination regimen had acceptable activity and good tolerability with maintenance of quality of life in this population of patients. In these patients, major grade III/IV toxicities were diarrhea (16.9%) and peripheral neuropathy (12.7%). In 2006, Bennouna et al. reported results of the regimen of OXA 130 mg/m2 given on day 1 combining the administration of oral tegafur–uracil 300 mg/m2/day and LV 90 mg/day from day 1 to 14 in a 21-day cycle (TEGAFOX) used as first-line treatment for patients with metastatic colorectal cancer [28]. Major grade III/IV toxicities caused by the abovementioned regimen were sensory neuropathy (15%), asthenia (13%) and diarrhea (11%). From our studies and review of the literature, OXA combining oral tegafur–uracil/LV appears to be a promising alternative for patients who are not suitable for or refuse to receive intravenous infusion of 5-FU.
Recently, several novel agents were evaluated for advanced gastric cancer. The V325 study [29] demonstrated that patients treated with doxcetaxel, CDDP and 5-FU (DCF) had better response and survival benefits than patients treated with CDDP and 5-FU (CF). DCF was suggested for use as a reference treatment for advanced gastric cancer. However, treatment with DCF resulted in a much higher percentage of hematologic toxicity than seen in the CF arm. This might be a limitation for use in conventional clinical practice. Recently, the final results from the REAL 2 study [30] showed that OXA and oral capcetabine could replace CDDP and low-dose infusion 5-FU given by pump for patients with esophagogastric junction cancer. To date, no clinical data could demonstrate whether one oral fluoropyridime is better than the other.
The major toxicity of tegafur–uracil/LV is diarrhea, and bone marrow toxicity is limited. The main limitations of capcetabine are diarrhea and hand-foot syndrome. S-1 causes fewer diarrheas but has higher incidence of bone marrow toxicity [31]. In the clinical practice, we can choose or change the different fluoropyridimes depending on physicians’ experiences, patient’s age and baseline symptoms. In addition, possible side effects that may happen during treatment should be closely monitored. The best measure is to integrate various aspects of tumor biology (i.e. thmidylate synthase and dihydropyrimidine dehydrogenase activity), which may predict sensitivity to particular drugs and allow individualization of therapy [31]. Further research is needed to identify the most active and convenient combination regimen with least toxic combinations for patients with advanced gastric cancer.
Ichikawa and sasaki presented a meta-analysis on correlation between tumor response to first-line chemotherapy and prognosis in patients with advanced gastric cancer [32]. They demonstrated the following: firstly, response rate might not be a valid surrogate for survival for the purpose of testing a new treatment; secondly, time to progression of roughly 5 months was considered a threshold point regardless of increasing response rate; thirdly, effective second-line chemotherapy may affect the outcome of patients with advanced gastric cancer. For regimens based on OXA and 5-FU, times to progression were almost more than 5 months. In the future, OXA-based chemotherapy with 5-FU may be considered one of the reference treatment for patients with advanced gastric cancer.
In conclusion, the combination of biweekly OXA and tegafur–uracil/LV in patients with advanced gastric cancer has acceptable activity and manageable toxicity. Efficacy data shown in current phase II trial was similar to the results reported in the previous studies using OXA and infusion 5-FU/LV. The safety profiles differed with a higher incidence of diarrhea but with less severe hematologic toxicities. Further study is warranted involving oxaliplatin combining different oral fluoropyrimidine drugs.
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Hundahl SA, Menck HR, Mansour EG, Winchester DP (1997) The National Cancer Data Base report on gastric carcinoma. Cancer 80:2333–2341
Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H, Heuman R (1997) Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 8:163–168
Wöhrer SS, Raderer M, Hejna M (2004) Palliative chemotherapy for advanced gastric cancer. Ann Oncol 15:1585–1595
Ohtus A (2005) Current status and future prospects of chemotherapy for metastatic gastric cancer: a review. Gastric Cancer 8:95–102
Maehara Y, Kakeji Y, Ohno S, Baba H, Sugimachi K (1997) Scientific basis for the combination of tegafur with uracil. Oncology (Huntington) 11(Suppl 10):14–18
Ho DH, Pazdur R, Covington W, Brown N, Huo YY, Lassere Y, Kuritani J (1998) Comparison of 5-fluorouracil pharmacokinetics in patients receiving continuous 5-fluorouracil infusion and oral uracil plus N1-(2′-tetrahydrofuryl)-5-fluorouracil. Clin Cancer Res 4:2085–2088
Namatame K, Sasaki E, Ko Y, Shimada K, Takahashi H, Nakano H, Midorikawa T, Makuuchi M, Kimura K, Kumada K (1993) A double-blind comparison of FUra plasma concentration by oral UFT vs continuous intravenous infusion (5-FU). Gan To Kagaku Ryoho 20:2417–2419 [in Japanese]
Takiuchi H, Ajani JA (1998) Uracil–tegafur in gastric carcinoma: a comprehensive review. J Clin Oncol 16:2877–2885
Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, Louvet C, Gespach C (1997) Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast, and ovarian cancers. Anticancer Drugs 8:876–885
Becouarn Y, Agostini C, Trufflandier N, Boulanger V (2001) Oxaliplatin: available data in non-colorectal gastrointestinal malignancies. Crit Rev Oncol Hematol 40:265–272
Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, Itzhaki M, Metzger G, N’Daw D, Vignoud J, Abad A, Francois E, Gamelin E, Marty M, Sastre J, Seitz JF, Ychou M (1996) Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol 7:95–98
Diaz-Rubio E, Sastre J, Zaniboni A, Labianca R, Cortes-Funes H, de Braud F, Boni C, Benavides M, Dallavalle G, Homerin M (1998) Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol 9:105–108
Kelly H, Goldberg RM (2005) Systemic chemotherapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol 23:4553–4560
Eriguchi M, Osada I, Fujii Y, Takeda Y, Yoshizaki I, Akiyama N, Yanagie H, Sekiguchi M, Kizu R, Matsushita H, Mathe G (1997) Pilot study for preoperative administration of 1-OHP to patients with advanced scirrhous type gastric cancer. Biomed Pharmacother 51:217–220
Louvet C, Coudray AM, Tournigand C, Prevost S, Raymond E, de Gramont A, Chazard M, Gespach C (2000) Synergistic antitumoral activity of combined UFT, folinic acid and oxaliplatin against human colorectal HT29 cell xenografts in athymic nude mice. Anticancer Drugs 11:579–582
Chen JS, Huang JS, Yang TS, Lin YC, Wang HM, Liau CT, Rau KM (2005) Phase I dosing-escalating study of oxaliplatin in combination with oral tegafur–uracil and leucovorin in patients with advanced gastric cancer. Anticancer Drugs 16:47–51
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2001) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, Francois E, Jacob JH, Levoir D, Taamma A, Rougier P, Cvitkovic E, de Gramont A (2002) Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 20:4543–4548
Al-Batran SE, Atmaca A, Hegewisch-Becker S, Jaeger D, Hahnfeld S, Rummel MJ, Seipelt G, Rost A, Orth J, Knuth A, Jaeger E (2004) Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol 22:658–663
Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, Chang CS, Chang JY, Chung CY, Kao WY, Hsieh RK, Cheng AL (2004) Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer 91:453–458
Lordick F, Lorenzen S, Stollfuss J, Vehling-Kaiser U, Kullmann F, Hentrich M, Zumschlinge R, Dietzfelbinger H, Thoedtmann J, Hennig M, Seroneit T, Bredenkamp R, Duyster J, Peschel C (2005) Phase II study of weekly oxaliplatin plus infusional fluorouracil and folinic acid (FUFOX regimen) as first-line treatment in metastatic gastric cancer. Br J Cancer 93:190–194
Jatoi A, Murphy BR, Foster NR, Nikcevich DA, Alberts SR, Knost JA, Fitch TR, Rowland KM Jr, North Central Cancer Treatment Group (2006) Oxaliplatin and capecitabine in patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction and gastric cardia: a phase II study from the North Central Cancer Treatment Group. Ann Oncol 17:29–34
Park YH, Kim BS, Ryoo BY, Yang SH (2006) A phase II study of capecitabine plus 3-weekly oxaliplatin as first-line therapy for patients with advanced gastric cancer. Br J Cancer 94:959–963
Koizumi W, Kurihara M, Nakano S, Haegawa K (2000) Phase II study of S1, a novel derivative of 5-fluorouracil, in advanced gastric cancer. Oncology 58:191–197
Feliu J, Vicent JM, Garcia-Giron C, Constela M, Fonseca E, Aparicio J, Lomas M, Anton-Aparicio L, Dorta FJ, Gonzalez-Baron M, Oncopaz Cooperative Group Associated Hospitals (2004) Phase II study of UFT and oxaliplatin in first-line treatment of advanced colorectal cancer. Br J Cancer 91:1758–1762
Rosati G, Cordio S, Tucci A, Blanco G, Bordonaro R, Reggiardo G, Manzione L (2005) Phase II trial of oxaliplatin and tegafur/uracil and oral folinic acid for advanced or metastatic colorectal cancer in elderly patients. Oncology 69:122–129
Bennouna J, Perrier H, Paillot B, Priou F, Jacob JH, Hebbar M, Bordenave S, Seitz JF, Cvitkovic F, Dorval E, Malek K, Tonelli D, Douillard JY (2006) A phase II study of oral uracil/ftorafur (UFT) plus leucovorin combined with oxaliplatin (TEGAFOX) as first-line treatment in patients with metastatic colorectal cancer. Br J Cancer 94:69–73
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol 31:4991–4997
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Schöffski P (2004) The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs 15:84–106
Ichikawa W, Sasaki Y (2006) Correlation between tumor response to first-line chemotherapy and prognosis in advanced gastric cancer patients. Ann Oncol 17:1665–1672
Acknowledgments
The authors thank all the patients who participated in this study. The study was supported by TTY Biopharm Co., Ltd, although the study was developed, written and originated from Chang Gung Memorial Hospital.
Conflict of interest statement
The authors declare no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Chen, JS., Rau, KM., Chen, YY. et al. A multiple-center phase II study of biweekly oxaliplatin and tegafur–uracil/leucovorin for chemonaive patients with advanced gastric cancer. Cancer Chemother Pharmacol 63, 819–825 (2009). https://doi.org/10.1007/s00280-008-0797-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0797-4