Summary
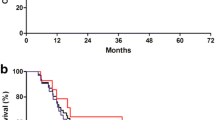
Purpose Chemotherapy remains the primary treatment for metastatic gastric/GEJ cancer but optimal agents and schedule remain controversial. This study examined the safety and efficacy of first-line Irinotecan, capecitabine (Xeloda®), and Oxaliplatin (IXO). Patients and Methods Eligible patients with HER2-unamplified/unknown, metastatic gastric/GEJ adenocarcinoma were treated with 21-day cycle IXO at dose level 1 (DL1: Day 1 O-100 mg/m2 & I-160 mg/m2 IV, Day 2–15 X-1900 mg/m2/day PO divided doses) or modified IXO (mIXO): Day 1 O-85 mg/m2 & I-120 mg/m2 IV, Day 2–15 X-1425 mg/m2/day PO divided doses). This Bryant and Day two-stage designed study had dual primary endpoints of objective response rate (ORR) and toxicity. Secondary endpoints were overall survival (OS) and progression-free survival (PFS). Results Fifty patients were enrolled and received a median of 7 cycles. After accrual of 9 patients at DL1, evaluable RR was 88% however dose limiting toxicity (DLT) rate was 56% thus doses were adjusted to mIXO. Fifteen patients accrued at mIXO had a RR of 60% and DLT rate of 13% allowing continuation to stage 2. Overall, 48 and 49 patients were evaluable for efficacy and safety, respectively, with ORR of 54% and DLTs in 24% of patients (DL1 = 56%; mIXO = 18%). Disease control rate was 85%. The most frequent grade 3/4 adverse events were diarrhea, neutropenia, fatigue, hypokalemia, and nausea. Median PFS and OS were 7.5 and 13.0 months, respectively, with a median follow-up of 9.7 months. Conclusion mIXO demonstrates promising ORR, PFS, OS, and acceptable toxicity compared to standard triplet regimens. IXO should be evaluated in phase III trials.



Similar content being viewed by others
References
World cancer report, S. B.W. and W. C.P., Editors. (2014) World Health Organization: France
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24(18):2903–2909. https://doi.org/10.1200/JCO.2005.05.0245
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358(1):36–46. https://doi.org/10.1056/NEJMoa073149
Van Cutsem E et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol 24(31):4991–4997. https://doi.org/10.1200/JCO.2006.06.8429
Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, Price T, Anderson H, Iveson T, Hickish T, Lofts F, Norman A (2002) Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20(8):1996–2004. https://doi.org/10.1200/JCO.2002.08.105
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates J, Nicolson M, Hickish T, O'Brien M, Iveson T, Watson M, Underhill C, Wardley A, Meehan M (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15(1):261–267. https://doi.org/10.1200/JCO.1997.15.1.261
Cavanna L, Artioli F, Codignola C, Lazzaro A, Rizzi A, Gamboni A, Rota L, Rodin C, Boni F, Iop A, Zaniboni A (2006) Oxaliplatin in combination with 5-fluorouracil (5-FU) and leucovorin (LV) in patients with metastatic gastric cancer (MGC). Am J Clin Oncol 29(4):371–375. https://doi.org/10.1097/01.coc.0000221358.57089.f2
Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, Park JN, Han JY, Kang JH, Lee KS, Cho JY (2004) A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol 15(9):1344–1347. https://doi.org/10.1093/annonc/mdh343
Kawakami T et al (2016) Efficacy and safety of irinotecan monotherapy as third line treatment for advanced gastric cancer. ASCO Meet Abstr 34(4_suppl):113
Lee JL, Kang YK, Kang HJ, Lee KH, Zang DY, Ryoo BY, Kim JG, Park SR, Kang WK, Shin DB, Ryu MH, Chang HM, Kim TW, Baek JH, Min YJ (2008) A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 99(4):584–590. https://doi.org/10.1038/sj.bjc.6604536
Oba M et al (2011) Irinotecan monotherapy offers advantage over combination therapy with irinotecan plus cisplatin in second-line setting for treatment of advanced gastric cancer following failure of fluoropyrimidine-based regimens. Oncol Lett 2(2):241–245. https://doi.org/10.3892/ol.2011.242
Park SH, Nam E, Park J, Cho EK, Shin DB, Lee JH, Lee WK, Chung M, Lee SI (2008) Randomized phase II study of irinotecan, leucovorin and 5-fluorouracil (ILF) versus cisplatin plus ILF (PILF) combination chemotherapy for advanced gastric cancer. Ann Oncol 19(4):729–733. https://doi.org/10.1093/annonc/mdm502
Lee J, Kang WK, Kwon JM, Oh SY, Lee HR, Kim HJ, Park BB, Lim HY, Han MJ, Park JO, Park YS (2007) Phase II trial of irinotecan plus oxaliplatin and 5-fluorouracil/leucovorin in patients with untreated metastatic gastric adenocarcinoma. Ann Oncol 18(1):88–92. https://doi.org/10.1093/annonc/mdl317
Okines AF et al (2009) Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 20(9):1529–1534. https://doi.org/10.1093/annonc/mdp047
Wohrer SS, Raderer M, Hejna M (2004) Palliative chemotherapy for advanced gastric cancer. Ann Oncol 15(11):1585–1595. https://doi.org/10.1093/annonc/mdh422
Maroun J et al (2007) Encouraging results from a phase I study of capecitabine (X), irinotecan (I) and oxaliplatin (O) as first-line therapy in patients (pts) with metastatic colorectal cancer (MCRC). ASCO Meet Abstr 25(18_suppl):4086
Masi G et al (2007) The combination of capecitabine (C), irinotecan (I) and oxaliplatin (O) (XELOXIRI) as first line treatment of metastatic colorectal cancer (MCRC): preliminary results of a pilot study by the Gruppo Oncologico Nord-Ovest (G.O.N.O.). ASCO Meet Abstr 25(18_suppl):4096
Samantas E et al (2005) Phase I dose-finding study of capecitabine (X) with escalating irinotecan (I) and oxaliplatin (O) in patients (pts) with previously treated solid tumors. ASCO Meet Abstr 23(16_suppl):3543
Maroun J, Marginean H, Jonker D, Cripps C, Goel R, Asmis T, Goodwin R, Chiritescu G (2017) A phase I study of Irinotecan, Capecitabine (Xeloda), and Oxaliplatin in patients with advanced colorectal Cancer. Clin Colorectal Cancer. https://doi.org/10.1016/j.clcc.2017.12.003
Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Filho SC, Majlis A, Assadourian S, van Cutsem E (2005) Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 23(24):5660–5667. https://doi.org/10.1200/JCO.2005.17.376
Cho EK, Lee WK, Im SA, Lee SN, Park SH, Bang SM, Park DK, Park YH, Shin DB, Lee JH (2005) A phase II study of epirubicin, cisplatin and capecitabine combination chemotherapy in patients with metastatic or advanced gastric cancer. Oncology 68(4–6):333–340
Ocvirk J, Reberšek M, Škof E, Hlebanja Z, Boc M (2012) Randomized prospective phase II study to compare the combination chemotherapy regimen epirubicin, cisplatin, and 5-fluorouracil with epirubicin, cisplatin, and capecitabine in patients with advanced or metastatic gastric cancer. Am J Clin Oncol 35(3):237–241. https://doi.org/10.1097/COC.0b013e31820dc0b0
Acknowledgements
We would like to thank the patients and their families. We also thank the research staff at participating sites, especially SeeSee Raynard and Kristine Young.
Funding
Funding for this investigator sponsored study was provided by Roche and Sanofi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Karen Mulder has consulting or advisory role from Novartis, Lilly and LEO Pharma, research funding from Amgen, Roche, Bayer, & Sanofi Canada; Christine Brezden-Masley has honoraria from Roche, consulting or advisory role from Roche, research funding from Roche; Michael Vickers has honoraria from Ipsen, Lilly, Novartis, & Celgene, consulting or advisory role from AstraZeneca; Jose Monzon has consulting or advisory role from Bristol-Myers Squibb, Celgene, Merck, Novartis, & Roche, speakers’ bureau from Bristol-Myers Squibb & Merck; Jennifer Spratlin has honoraria from Lilly, Celgene Taiho & Astellas, consulting or advisory role from Lilly, Celgene, Taiho & Astellas, and research funding from Sanofi, Roche, & Celgene. All other authors have nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Lui, A., Mulder, K., Brezden-Masley, C. et al. A multicentre, open-label phase II study of Irinotecan, capecitabine (Xeloda®), and Oxaliplatin (IXO) as first-line treatment in patients with metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma. Invest New Drugs 36, 674–682 (2018). https://doi.org/10.1007/s10637-018-0599-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0599-4