Abstract
Purpose
Pharmacokinetic data on fenretinide (4-HPR) are scant, thus limiting the rational use of the drug. We investigated the pharmacokinetics of 4-HPR and its active metabolite 4-oxo-fenretinide (4-oxo-4-HPR).
Experimental design
Pharmacokinetics were assessed in 18 children (3 for each dose) with neuroblastoma who received oral 4-HPR once daily for 28 days at the doses of 100, 300, 400, 600, 1,700 and 4,000 mg/m2/day. 4-HPR and 4-oxo-4-HPR were determined by HPLC in plasma collected up to 48 h after the first and 28th administration.
Results
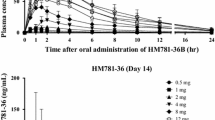
After single administration, 4-HPR mean C max ranged from 0.9 to 6.6 μM and these concentrations roughly doubled at steady state (range 1.6–14.5 μM). 4-HPR mean t 1/2 was 22 h. 4-HPR pharmacokinetics were linear in the dose range 100–1,700 mg/m2; less than dose-proportional increase in exposure was found at 4,000 mg/m2. At steady state, pharmacologically relevant plasma concentrations (range 0.7–10 μM and 0.4–5 μM for 4-HPR and 4-oxo-4-HPR, respectively) were maintained during the 24 h dosing interval in the dose range 300–4,000 mg/m2.
Conclusions
4-HPR pharmacokinetics supports once-daily dosing. Steady state concentrations of 4-HPR and 4-oxo-4-HPR in children with neuroblastoma are in line with those found to have in vitro growth inhibitory effects in neuroblastoma cells.


Similar content being viewed by others
References
Nagy L, Thomazy VA, Heyman RA, Davies PJ (1988) Retinoid-induced apoptosis in normal and neoplastic tissues. Cell Death Differ 5:11–19
Chiesa F, Tradati N, Grigolato R, Crose N, Cavadini E, Formelli F et al (2005) Randomized trial of fenretinide (4-HPR) to prevent recurrences, new localizations and carcinoma in patients operated on for oral leukoplakia: long-term results. Int J Cancer 115:625–629
Veronesi U, De Palo G, Marubini E, Costa A, Formelli F, Mariani L et al (1999) Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst 91:1847–1856
De Palo G, Mariani L, Camerini T, Marubini E, Formelli F, Pasini B et al (2002) Effect of fenretinide on ovarian carcinoma occurence. Gynecol Oncol 86:24–27
Vaishampayan U, Heilbrun LK, Parchment RE, Jain V, Zwiebel J, Boinpally RR et al (2005) Phase II trial fenretinide in advanced renal carcinoma. Invest New Drugs 23:179–185
Villani MG, Appierto V, Cavadini E, Bettiga A, Prinetti A, Clagett-Dame M et al (2006) 4-oxo-fenretinide, a recently identified fenretinide metabolite, induces marked G2-M cell cycle arrest and apoptosis in fenretinide-sensitive and fenretinide-resistant cell lines. Cancer Res 66:3238–3247
Clifford JL, Menter DG, Wang M, Lotan R, Lippman SM (1999) Retinoid receptor-dependent and—independent effects of N-(4-hydroxyphenyl) retinamide in F9 embryonal carcinoma cells. Cancer Res 59:14–18
Formelli F, Clerici M, Campa T, Di Mauro MG, Magni A, Mascotti G et al (1993) Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J Clin Oncol 11:2036–2042
Appierto V, Cavadini E, Pergolizzi R, Cleris L, Lotan R, Canevari S, et al (2001) Decrease in drug accumulation and in tumor aggressiveness marker expression in a fenretinide-induced resistant ovarian tumor cell lines. Br J Cancer 84:1528–1534
Villani MG, Appierto V, Cavadini E, Valsecchi M, Sonnino S, Curley RW et al (2004) Identification of the fenretinide metabolite 4-oxo-fenretinide present in human plasma and formed in human ovarian carcinoma cells through induction of cytochrome P450 26A1. Clin Cancer Res 10:6265–6275
Veronesi U, Mariani L, Decensi A, Formelli F, Camerini T, Miceli R et al (2006) Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol 17:1065–1071
Otterson GA, Lavelle J, Villalona-Calero MA, Shah M, Wei X, Chan KK et al (2005) A phase I clinical and pharmacokinetic study of fenretinide combined with paclitaxel and cisplatin for refractory solid tumors. Invest New Drugs 23:555–562
Puduvalli VK, YungWK, Hess KR, Kuhn JG, Groves MD, Levin VA et al (2004) Phase II study of fenretinide (NSC 374551) in adults with recurrent malignant gliomas: a north American brain tumor consortium study. J Clin Oncol 22:4282–4289
Villablanca J, Krailo D, Ames MM, Reid JM, Reaman GH, Reynolds P (2006) Phase I trial of oral fenretinamide in children with high-risk solid tumors: a report from the children’s oncology group (CCG 09709). J Clin Oncol 24:3423–3430
Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR et al (2003) Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res 9:2032–2039
Doose DR, Minn FL, Stellar S, Nayak RK (1992) Effects of meals and meal composition on the bioavailability of fenretinide. J Clin Pharmacol 32:1089–1095
Singletary SE, Atkinson EN, Hoque A, Sneige N, Sahin AA, Fritsche HA Jr et al (2002) Phase II trial of N-(4-Hydroxyphenyl)retinamide and tamoxifen administration before definitive surgery for breast neoplasia. Clin Cancer Res 8:2835–2842
Sabici A, Modiano MR, Lee JJ, Peng YM, Xu MJ, Villar H et al (2003) Breast tissue accumulation of retinamides in a randomized short-term study of fenretinide. Clin Cancer Res 9:2400–2405
Follen M, Atkinson EN, Schottenfeld D, Malpica A, West L, Lippman S et al (2001) A randomized clinical trial of 4-Hydroxyphenylretinamide for high-grade squamous intraepithelial lesions of the cervix. Clin Cancer Res 7:3356–3365
Kurie JM, Lee JS, Khuri FR, Mao L, Morice RC, Lee JJ et al (2000) N-(4-Hydroxyphenyl)retinamide in the chemoprevention of squamous metaplasia and dysplasia of the bronchial epithelium. Clin Cancer Res 6:2973–2979
Thaller C, Shalev M, Frolov A, Eichele G, Thompson TC, Williams RH et al (2000) Fenretinide therapy in prostate cancer: effects on tissue and serum retinoid concentration. J Clin Oncol 22:3804–3808
Conley B, O’Shaughnessy J, Prindiville S, Lawrence J, Chow C, Jones E et al (2000) Pilot trial of the safety tolerability, and retinoid levels of N-(4-hydroxyphenyl) retinamide in combination with tamoxifen in patients at high risk for developing invasive breast cancer. J Clin Oncol 18:275–283
Colombo N, Formelli F, Cantù MG, Parma G, Gasco M, Argusti A et al (2006) A phase I–II preoperative biomarker trial of fenretinide in ascitic ovarian cancer. Cancer Epidemiol Biomarkers Prev 15:1914–1919
Rowland ML, Tozer TN (1995) Clinical pharmacokinetics concepts and application, 3rd edn. Lippincott Williams and Wilkins, Baltimore
US FDA Guidance for Industry (1998) General considerations for pediatric pharmacokinetic studies for drugs and biological products. US FDA, Rockville
Acknowledgment
Grant support: Associazione Italiana per la Ricerca sul Cancro, Milan, Italy and Gateway for Cancer Research (formerly Cancer Treatment Research Foundation) Grant G-96-131.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Formelli, F., Cavadini, E., Luksch, R. et al. Pharmacokinetics of oral fenretinide in neuroblastoma patients: indications for optimal dose and dosing schedule also with respect to the active metabolite 4-oxo-fenretinide. Cancer Chemother Pharmacol 62, 655–665 (2008). https://doi.org/10.1007/s00280-007-0649-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0649-7