Abstract
Purpose
The pathobiology of alimentary tract (AT) mucositis is complex and there is limited information about the events which lead to the mucosal damage that occurs during cancer treatment. Various transcription factors and proinflammatory cytokines are thought to play important roles in pathogenesis of mucositis. The aim of this study was to determine the expression of nuclear factor-κB (NF-κB), tumor necrosis factor (TNF) and interleukins-1β (IL-1β) and -6 (IL-6) in the AT following the administration of the chemotherapeutic agent irinotecan.
Methods
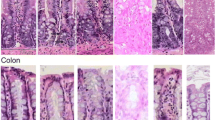
Eighty-one female dark Agouti rats were assigned to either control or experimental groups according to a specific time point. Following administration of irinotecan, rats were monitored for the development of diarrhoea. The rats were killed at times ranging from 30 min to 72 h after administration of irinotecan. Oral mucosa, jejunum and colon were collected and standard immunohistochemical techniques were used to identify NF-κB, TNF, IL-1β and IL-6 within the tissues. Sections were also stained with haematoxylin and eosin for histological examination.
Results
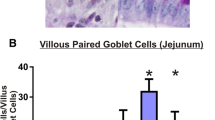
Irinotecan caused mild to moderate diarrhoea in a proportion of the rats that received the drug. Altered histological features of all tissues from rats administered irinotecan were observed which included epithelial atrophy in the oral mucosa, reduction of villus height and crypt length in the jejunum and a reduction in crypt length in the colon. Tissue staining for NF-κB, TNF and IL-1β and IL-6 peaked at between 2 and 12 h in the tissues examined.
Conclusions
This is the first study to demonstrate histological and immunohistochemical evidence of changes occurring concurrently in different sites of the AT following chemotherapy. The results of the study provide further evidence for the role of NF-κB and associated pro-inflammatory cytokines in the pathobiology of AT mucositis. The presence of these factors in tissues from different sites of the AT also suggests that there may be a common pathway along the entire AT causing mucositis following irinotecan administration.








Similar content being viewed by others
References
Alimonti A, Gelibter A, Pavese I et al (2004) New approaches to prevent intestinal toxicity of irinotecan-based regimens. Cancer Treat Rev 30:555–562
Anthony L, Bowen J, Garden A et al (2006) New thoughts on the pathobiology of regimen-related mucosal injury. Support Care Cancer 14:516–518
Bowen JM, Gibson RJ, Keefe DM et al (2005) Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37:56–62
Cool JC, Dyer JL, Xian CJ et al (2005) Pre-treatment with insulin-like growth factor-I partially ameliorates 5-fluorouracil-induced intestinal mucositis in rats. Growth Hormone IGF Res 15:72–82
Elting LS, Cooksley C, Chambers MS et al (2003) The burdens of cancer therapy: clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98:1531–1539
Gibson R, Keefe D (2006) Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Supportive Care in Cancer 1–11
Gibson RJ, Bowen JM, Inglis MRB et al (2003) Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 18:1095–1100
Gibson RJ, Cummins AG, Bowen JM et al (2006) Apoptosis occurs early in the basal layer of the oral mucosa following cancer chemotherapy. Asian Pacific J Clin Oncol 2:39–49
Gibson RJ, Keefe DM, Thompson FM et al (2002) Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci 47:2751–2757
Iyer L, King CD, Whitington PF et al (1998) Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 101:847–854
Junqueira L, Carneiro J, Long J (2005) Basic histology, 11th edn. McGraw-Hill, Lange
Kawahara M (2006) Irinotecan in the treatment of small cell lung cancer: a review of patient safety considerations. Expert Opin Drug Saf 5:303–312
Keefe DM, Gibson RJ, Hauer-Jensen M (2004) Gastrointestinal mucositis. Semin Oncol Nurs 20:38–47
Keefe DMK (2004) Gastrointestinal mucositis: a new biological model. Support Care Cancer 12:6–9
Keefe DMK (2006) Mucositis guidelines: what have they acheived and where to from here? Support Care Cancer 14:489–491
Keefe DMK, Brealey J, Goland GJ et al (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47:632–637
Krajewska M, Wang HG, Krajewski S et al (1997) Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res 57:1605–13
Krajewska M, Zapata JM, Meinhold-Heerlein I et al (2002) Expression of Bcl-2 family member Bid in normal and malignant tissues. Neoplasia 4:129–40
Krajewski S, Krajewska M, Reed JC (1996) Immunohistochemical analysis of in vivo patterns of Bak expression, a proapoptotic member of the Bcl-2 protein family. Cancer Res 56:2849–2855
Logan RM, Gibson RJ, Sonis ST et al (2007) Nuclear factor-kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol 43:395–401
Logan RM, Stringer AM, Bowen JM et al. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev. doi:10.1016/j.ctrv.2007.03.001
Melo M, Brito G, Soares R et al. Role of cytokines (TNF-α, IL-1β and KC) in the pathogenesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomide. Cancer Chemotherapy and Pharmacology. doi:10.1007/s00280-007- 0534-4
Mocellin S, Rossi CR, Pilati P et al (2005) Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev 16:35–53
Nakamura K, Honda K, Mizutani T et al (2006) Novel strategies for the treatment of inflammatory bowel disease: selective inhibition of cytokines and adhesion molecules. World J Gastroenterol 12:4628–4635
Pico J-L, Avila-Garavito A, Naccache P (1998) Mucositis: its occurrence, consequences, and treatment in the oncology setting. Oncologist 3:446–451
Podolsky DK (2002) Inflammatory Bowel Disease. N Engl J Med 347:417–429
Scully C, Epstein J, Sonis S (2003) Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: part 1, pathogenesis and prophylaxis of mucositis. Head Neck 25:1057–70
Sonis ST (1998) Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 34:39–43
Sonis ST (2002) The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with antineoplastic therapy. Crit Rev Oral Biol Med 13:380–390
Sonis ST, Elting LS, Keefe D et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025
Stringer AM, Gibson RJ, Logan RM et al (2007) Chemotherapy-Induced diarrhoea is associated with changes in the luminal environment in the DA rat. Exp Biol Med 232:96–106
Tallman MN, Miles KK, Kessler FK et al (2007) The contribution of intestinal UDP-glucuronosyltransferases in modulating 7-ethyl-10-hydroxy-camptothecin (SN-38)-induced gastrointestinal toxicity in rats. J Pharmacol Exp Ther 320:29–37
Wang L, Walia B, Evans J et al (2003) IL-6 Induces NF-{kappa}B Activation in the intestinal epithelia. J Immunol 171:3194–3201
Yeoh ASJ, Bowen JM, Gibson RJ et al (2005) Nuclear factor- kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the irradiated colorectum is associated with subsequent histopathological changes. Int J Rad Oncol Biol Phys 63:1295–1303
Acknowledgments
This project was supported by a research grant awarded by the Royal Adelaide Hospital Research Committee and the Australian Dental Research Foundation Incorporated. Dr. Rachel Gibson was supported by a Cancer Council South Australia Research Fellowship; Dr. Joanne Bowen and Ms. Andrea Stringer were supported by a Dawes Research Scholarship. Assistance with the statistical analysis for this study was provided by Mr. Thomas Sullivan from the Department of Public Health, The University of Adelaide. The authors also acknowledge Ms Marjorie Quinn, Oral Pathology, School of Dentistry, The University of Adelaide for her assistance in cutting sections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest statement: None of the authors are aware of any potential conflicts of interest that may have inappropriately influenced or biased this work.
Rights and permissions
About this article
Cite this article
Logan, R.M., Gibson, R.J., Bowen, J.M. et al. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 62, 33–41 (2008). https://doi.org/10.1007/s00280-007-0570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0570-0