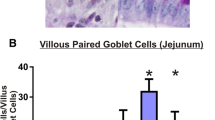
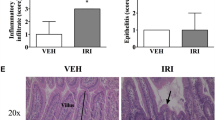
Abstract
Purpose
Chemotherapy-induced mucositis is characterised by damage to mucous membranes throughout the alimentary tract. This study aims to investigate the expression of cell adhesion molecules (CAMs) following treatment with irinotecan.
Methods
Dark agouti rats received a single dose of 175 mg/kg irinotecan and sacrificed at various time points after treatment. Picro-sirius red staining indicated an increase in collagen around crypts from 24 h in both small and large intestinal regions and this diminished at the later time points. CAMs E-cadherin, P-selectin, E-selectin and integrin-α1 were examined using immunohistochemistry.
Results
E-cadherin was significantly elevated in jejunal crypts at the time of maximal tissue damage (48 h), while it decreased at the healing phase (96 h) in both jejunum and colon. P-selectin expression decreased significantly in the jejunum following irinotecan. Crypt expression of E-selectin was significantly elevated in the healing phase of mucositis (96 h). Integrin-α1 expression was significantly altered during the time course in the villus (p = 0.0032) and lamina propria (p = 0.039).
Conclusions
Irinotecan induced a significant alteration in CAM expression in the jejunum and colon. Changes in adhesion molecule expression may have a direct impact on the loss of mucosal layer integrity seen in mucositis.






Similar content being viewed by others
References
Sonis S (2007) Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 5:s3–11
Gibson R, Bowen J, Cummins A, Keefe D (2005) Relationship between dose of methotrexate, apoptosis, p53/p21 expression and intestinal crypt proliferation in the rat. Clin Exp Med 4:188–195
Yeoh A, Bowen J, Gibson R, Keefe D (2005) Nuclear factor κB (NFκB) and cyclooxygenase-2 (COX-2) expression in the irradiated colorectum is associated with subsequent histopathological changes. Int J Radiat Oncol Biol Phys 63:1295–1303
Sonis S (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284
Keefe D, Schubert M, Elting L, Sonis S, Epstein J, Raber-Durlacher J, Migliorati C, McGuire D, Hutchins R, Peterson D (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Keefe D (2004) Gastrointestinal mucositis: a new biological model. Support Care Cancer 12:6–9
Murphy B (2007) Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J Support Oncol 5:13–21
Sonis S (2004) A biological approach to mucositis. J Support Oncol 2:21–32
Logan R, Gibson R, Bowen J, Stringer A, Sonis S, Keefe D (2008) Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 62:33–41
Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R (2001) Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293:293–297
Stringer A, Gibson R, Bowen J, Ashton K, Logan R, Al-Dasooqi N, Yeoh A, Keefe D (2009) Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol 90:489–499
Stringer A, Gibson R, Logan R, Bowen J, Yeoh A, Keefe D (2009) Gastrointestinal microflora and mucins play a role in the development of 5-fluorouracil-induced gastrointestinal mucositis in rats. Exp Biol Med 234:430–441
Stringer A, Gibson R, Bowen J, Keefe D (2009) Chemotherapy-induced changes to microflora: evidence and implications of change. Curr Drug Metab 10:79–83
Stringer A, Gibson R, Logan R, Bowen J, Yeoh A, Keefe D (2008) Faecal microflora and β-glucuronidase expression are altered in an irinotecan-induced diarrhoea model in rats. Cancer Biol Ther 7:1919–1925
Al-Dasooqi N, Bowen J, Gibson R, Logan R, Stringer A, Keefe D (2011) Irinotecan-induced alterations in intestinal cell kinetics and extracellular matrix component expression in the dark agouti rat. Int J Exp Pathol 92:357–365
Michael H, Gordon I, and Wojciech P (2003) Histology: a text and atlas. 4th edition ed. Lippincott Williams & Wilkins: Philadelphia, US
Yurchenco P, Schittny J (1990) Molecular architecture of basement membranes. FASEB J 4:1577–1590
Beaulieu J (1997) Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog Histochem Cytochem 31:1–78
Meredith J, Fazeli B, Schwartz M (1993) The extracellular matrix as a cell survival factor. Mol Biol Cell 4:953–961
Carroll K, Wong T, Drabik D, Chang E (1988) Differentiation of rat small intestinal epithelial cells by extracellular matrix. Am J Phys 254:g355–g360
Gibson RJ, Keefe DM, Clarke JM, Regester GO, Thompson FM, Goland GJ, Edwards BG, Cummins AG (2002) The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol 50(1):53–58
Gibson RJ, Keefe DM, Thompson FM, Clarke JM, Goland GJ, Cummins AG (2002) Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci 47(12):2751–2757
Gibson RJ, Bowen JM, Inglis MR, Cummins AG, Keefe DM (2003) Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 18(9):1095–1100
Gibson RJ, Bowen JM, Alvarez E, Finnie J, Keefe DM (2007) Establishment of a single-dose irinotecan model of gastrointestinal mucositis. Chemotherapy 53(5):360–369
Logan R, Stringer A, Bowen J, Yeoh A, Gibson R, Sonis S, Keefe D (2007) The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev 33:448–460
Bowen J, Gibson R, Keefe D, Cummins A (2005) Cytotoxic chemotherapy up-regulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37:56–62
Gibson R, Bowen J, Inglis M, Cummins A, Keefe D (2003) Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 18:1095–1100
Ma M, McLeod H (2003) Lessons learned from the irinotecan metabolic pathway. Curr Med Chem 10:41–49
Gibson R, Bowen J, Alvarez E, Finnie J, Keefe D (2007) Establishment of a single-dose irinotecan model of gastrointestinal mucositis. Chemotherapy 53:360–369
Potten C, Booth C, Pritchard D (1997) The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol 78:219–243
Orberg J, Klein L, Hiltner A (1982) Scanning electron microscopy of collagen fibers in intestine. Connect Tissue Res 9:187–193
Lalla R, Bowen J, Barasch A, Elting L, Epstein J, Keefe D, McGuire D, Nicolatou-Galitis O, Peterson D, Raber-Durlacher J, Sonis S, Elad S, (MASCC/ISOO) TMGLGotMAoSCiCaISoOO (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120:1453–1461
Bruewer M, Luegering A, Kucharzik T, Parkos C, Madara J, Hopkins A, Nusrat A (2003) Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171:6164–6172
Wardill H, Bowen J, and Gibson R (2013) Irinotecan disrupts tight junction protein occludin in the rat small intestine in Multinational Association for Supportive Care in Cancer. Journal of Supportive Care in Cancer: Berlin, Germany
Walzog B, Gaehtgens P (2000) Adhesion molecules: the path to a new understanding of acute inflammation. Physiology 15:107–113
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were approved by the Animal Ethics Committees of the Institute of Medical and Veterinary Sciences and the University of Adelaide and complied with the National Health and Medical Research Council (Australia) Code of Practice for Animal Care in Research and Training (2004).
Conflict of interest
Dr. Noor Al-Dasooqi was supported by a Postgraduate Research Fellowship from the Centre for Clinical Research Excellence; Dr. Joanne M Bowen was supported by a Postdoctoral Research Fellowship from the National Health and Medical Research Council; Professor Dorothy Keefe is the Cancer Council SA Chair of Cancer Medicine; and A/Prof Rachel J Gibson received funding from Cure Cancer and Cancer Australia. Assistance with the statistical analysis for this study was provided by Mr. Thomas Sullivan from the Department of Public Health, The University of Adelaide. There are no conflicts of interest to declare. The authors have full control of all primary data and we allow the journal to review the data if requested.
Rights and permissions
About this article
Cite this article
Al-Dasooqi, N., Bowen, J., Bennett, C. et al. Cell adhesion molecules are altered during irinotecan-induced mucositis: a qualitative histopathological study. Support Care Cancer 25, 391–398 (2017). https://doi.org/10.1007/s00520-016-3413-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3413-x