Abstract
Purpose
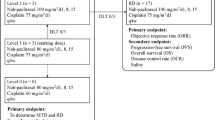
This trial was conducted to determine the maximum tolerated dose (MTD), principal toxicity, and recommended dose for phase II study of the combination of nedaplatin and weekly paclitaxel in patients with advanced non-small cell lung cancer (NSCLC).
Methods
Patients with previously untreated NSCLC, either stage IIIB with pleural effusion or stage IV, were eligible if they had a performance status of 0–2, were 75 years or younger, and had adequate organ function. The respective doses of nedaplatin (day 1) and weekly paclitaxel (days 1, 8, and 15) studied were 80/60, 80/70, 80/80, 80/90, and 100/90 (mg m−2), repeated every 4 weeks.
Results
From May 2004 through June 2005, 21 patients (18 men and 3 women; median age, 63 years; age range, 53–75 years) were enrolled. The MTD was determined to be 100 mg m−2 of nedaplatin and 90 mg m−2 of weekly paclitaxel. Dose-limiting toxicities at the MTD were neutropenic fever and hepatic dysfunction. We recommend doses of 80 mg m−2 of nedaplatin and 90 mg m−2 of weekly paclitaxel for phase II study. Grade 3–4 hematologic toxicities included neutropenia in 29% of patients, thrombocytopenia in 0%, and anemia in 5%. Although the most frequent non-hematologic toxicity was hepatic dysfunction, all cases were only mildly to moderately severe. Although two patients had grade 3 or 4 pulmonary toxicity due to Pneumocystis carinii pneumonia, these patients recovered after receiving trimetoprim-sulfamethoxazole, steroid therapy, and supplemental oxygen. There were no treatment-related deaths. The overall response rate was 19.0% (95% confidence interval, 5.4–41.9%), and all responses were in patients receiving the recommended doses. The median dose-intensities for nedaplatin and paclitaxel were 91.6 and 87.1%, respectively, of the planned doses.
Conclusion
This combination chemotherapy is active and well tolerated and warrants phase II study.
Similar content being viewed by others
References
Akerley W, Glantz M, Choy H, Rege V, Sambandam S, Joseph P, Yee L, Rodrigues B, Wingate P, Leone L (1998) Phase I trial of weekly paclitaxel in advanced lung cancer. J Clin Oncol 16:153–158
Belani CP, Barstis J, Perry MC, La Rocca RV, Nattam SR, Rinaldi D, Clark R, Mills GM (2003) Multicenter randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol 21:2933–2939
Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R, Taraboletti G (1996) The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res 2:1843–1849
Bookman MA, Kloth DD, Kover PE, Smolinski S, Ozols RF (1997) Short-course intravenous prophylaxis for paclitaxel-related hypersensitivity reactions. Ann Oncol 8:611–614
Furuse K, Fukuoka M, Asamoto H, Niitani H, Kimura I, Sakuma A, Yamaguchi Y (1992) A randomized comparative study of 254-S plus Vindesin (VDS) versus Cisplatin plus VDS in patients with advanced non-small cell lung cancer. Jpn J Cancer Chemothr 19:1019–1026 (in Japanese)
Georgiadis MS, Russell EK, Gazdar AF, Johnson BE (1997) Paclitaxel cytotoxicity against human lung cancer cell lines increases with prolonged exposure durations. Clin Cancer Res 3:449–454
Kelly K, Crowley J, Burn PA Jr, Presant CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR, Moore DF, Israel VK, Livingston RB, Gandara DR (2001) Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patient with advanced non-small-cell lung cancer: a southwest oncology group trial. J Clin Oncol 19:3210–3218
Klastersky J, Sculier JP, Lacroix H, Dabouis G, Bureau G, Libert P, Richez M, Ravez P, Vandermoten G, Thiriaux J (1990) A randomized study comparing cisplatin or carboplatin with etoposide in patients with advanced non-small-cell lung cancer: European Organization for Research and Treatment of Cancer Protocol 07861. J Clin Oncol 8:1556–1562
Koizumi T, Kubo K, Shinozaki S, Koyama S, Amari T, Hayano T, Fujimoto K, Kobayashi T, Sekiguchi M, Sakai R (1993) Pharmacokinetic evaluation of (glycolao-O,O′) diammine platinum (II) in lung lymph in sheep. Jpn J Cancer Res 84:468–473
Koumakis G, Demiri M, Barbounis V, Vassilomanolakis M, Gontikakis E, Pamouksoglou P, Dahabre J, Efremidis AP (2002) Is weekly paclitaxel superior to paclitaxel given every 3 weeks? Results of phase II trial. Lung Cancer 35:315–317
Lau D, Leigh B, Gandara D, Edelman M, Morgan R, Israel V, Lara P, Wilder R, Ryu J, Doroshow J (2001) Twice-weekly paclitaxel and weekly carboplatin with concurrent thoracic radiation followed by carboplatin/paclitaxel consolidation for stage III non-small-cell lung cancer: a California cancer consortium phase II trial. J Clin Oncol 19:442–447
Lau D, Ryu J, Gandara D, Morgan R, Doroshow J, Wilder R, Leigh B (1999) Concurrent twice-weekly paclitaxel and thoracic irradiation for stage III non-small cell lung cancer. Semin Radiat Oncol 9:117–120
Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, Mitchell JB (1993) Cytotoxic studies of paclitaxel (Taxol) in human tumor cell lines. Br J Cancer 68:1104–1109
Oshita F, Yamada K, Kato Y, Ikehara M, Noda K, Tanaka G, Nomura I, Suzuki R, Saito H (2003) Phase I/II study of escalating doses of nedaplatin in combination with irinotecan for advanced non-small cell lung cancer. Cancer Chemother Pharmacol 52:73–78
Ranson M, Davidson N, Nicolson M, Falk S, Carmichael J, Lopez P, Anderson H, Gustafson N, Jeynes A, Gallant G, Washington T, Thatcher N (2000) Randomized trial of paclitaxel plus supportive care versus supportive care for patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 92:1074–1080
Reckzeh B, Merte H, Pfluger KH, Pfab R, Wolf M, Havemann K (1996) Severe lymphocytopenia and interstitial pneumonia in patients treated with paclitaxel and simultaneous radiotherapy for non-small-cell lung cancer. J Clin Oncol 14:1071–1076
Reshkin SJ, Bellizzi A, Cardone RA, Tommasino M, Casavola V, Paradiso A (2003) Paclitaxel induces apoptosis via protein kinase A- and p38 mitogen-activated protein-dependent inhibition of the Na(+)/H(+) exchanger (NHE) isoform 1 in human breast cancer cells. Clin Cancer Res 9:2366–2373
Sandler A, Gray R, Perry M, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. NEJM 355:2542–2550
Sasaki Y, Saijo N, Tamura T (1987) Comparison of the antitumor activity of cisplatin and its derivatives with special stress on the pharmacokinetics of active form of drugs in the plasma determined by colony assay. Proc Am Soc Clin Oncol 6:34
Shirai T, Hirose T, Noda M, Ando K, Ishida H, Hosaka T, Ozawa T, Okuda K, Ohnishi T, Ohmori T, Horichi N, Adachi M (2006) Phase II study of the combination of gemcitabine and nedaplatin for advanced non-small cell lung cancer. Lung Cancer 52:181–187
Schuette W, Blankenburg T, Guschall W, Dittrich I, Schroeder M, Schweisfurth H, Chemaissani A, Schumann C, Dickgreber N, Appel T, Ukena D (2006) Muliticenter randomized trial for stage IIIB/IV non-small-cell lung cancer using every 3-week versus weekly paclitaxel/ carboplatin. Clin Lung Cancer 7:338–343
Seidman AD, Hudis CA, Albanel J, Tong W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L (1998) Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 16:3353–3361
Sekine I, Nokihara H, Horiike A, Yamamoto N, Kunitoh H, Ohe Y, Tamura T, Kodama T, Saijo N (2004) Phase I study of cisplatin analogue nedaplatin (254-S) and paclitaxel in patients with unresectable squamous cell carcinoma. Br J Cancer 90:1152–1158
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH (2002) Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell cancer. N Engl J Med 346:92–98
Shiratori O, Kanai H, Uchida N (1985) (eds) Recent advances in chemotherapy: antitumor activity of 254-S, a platinum complex, in rodents. University of Tokyo Press, Tokyo
Socinski MA, Ivanova A, Bakri K, Wall J, Baggstrom MQ, Hensing TA, Mears A, Tynan M, Beaumont J, Peterman H, Niell HB (2006) A randomized phase II trial comparing every 3-weeks carboplatin/paclitaxel with every 3-weeks carboplatin and weekly paclitaxel in advanced non-small-cell lung cancer. Ann Oncol 17:104–109
Tolaney SM, Partridge AH, Sheib RG, Burstein HJ, Winer EP (2006) Pneumocystis carinii pneumonia during dose-dense chemotherapy for breast cancer. J Clin Oncol 24:5330–5331
Yamada H, Uchida N, Maekawa R, Yoshioka T (2001) Sequence-dependent antitumor efficacy of combination chemotherapy with nedaplatin, a newly developed platinum, and paclitaxel. Cancer Lett 172:17–25
Yoshiike F, Koizumi T, Kitaguchi Y Hatayama O, Yasuo M, Sasabayashi M, Wakamatsu H, Fujimoto K, Kubo K (2005) Phase I trial of nedaplatin and paclitaxel for patients with non-small cell lung cancer. J Chemother 17:550–554
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okuda, K., Hirose, T., Ishida, H. et al. Phase I study of the combination of nedaplatin and weekly paclitaxel in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol 61, 829–835 (2008). https://doi.org/10.1007/s00280-007-0540-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0540-6