Abstract
Background
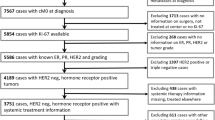
The aim of the study was to identify reliable predictive biological markers for treatment outcome following neoadjuvant adriamycin/docetaxel (AT) chemotherapy in locally advanced breast cancer patients.
Materials and methods
This study was a phase II study on AT neoadjuvant chemotherapy in locally advanced breast cancer patients. Patients received 50 mg/m2 of doxorubicin intravenously (IV) over 15 min followed by docetaxel 75 mg/m2 infused over 1 h, repeated every 3 weeks for three cycles. Surgery was performed within 3–4 weeks following the last cycle of chemotherapy. We analyzed the pre-treatment and post-treatment expression levels of ER, PgR, HER-2, Ki-67 proliferation index, and p53 and examined the correlation between the markers and clinical parameters with treatment response, overall survival and relapse-free survival following neoadjuvant treatment.
Results
From July 2001 to September 2004, 61 patients were enrolled. The meaningful parameters adversely influencing survival were post-treatment ER(−) status (P = 0.013) and post-treatment Ki-67 index above 1.0% (P = 0.013). At the multivariate level, the post-treatment Ki-67 proliferation index ≤ 1.0 was the only meaningful prognostic factor for better survival (P = 0.033). Notably, tumors with Ki-67 index ≤ 1.0 were more likely to express ER with statistical significance (P = 0.002). Tumors with ER(+) and Ki-67 index ≤ 1.0 showed the highest survival rate, followed by ER(+) and Ki-67 index > 1.0%, ER(−) and Ki-67 ≤ 1.0%, and ER(−) and Ki-67 > 1.0% with the worst survival (P = 0.033).
Conclusion
Collectively, post-treatment ER status and Ki-67 proliferation index were prognostic of overall survival following neoadjuvant AT chemotherapy.


Similar content being viewed by others
References
Schwartz GF, Hortobagyi GN (2004) Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, 26–28 April 2003, Philadelphia, Pennsylvania. Cancer 100:2512–2532
Bonadonna G, Valagussa P (1996) Primary chemotherapy in operable breast cancer. Semin Oncol 23:464–474
Ellis PA, Smith IE (1996) Primary chemotherapy for early breast cancer. Cancer Treat Rev 22:437–450
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, Dorval T, Palangie T, Jouve M, Beuzeboc P et al (1994) Neoadjuvant versus adjuvant chemotherapy in pre-menopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer 30A:645–652
Cunningham JD, Weiss SE, Ahmed S, Bratton JM, Bleiweiss IJ, Tartter PI, Brower ST (1998) The efficacy of neoadjuvant chemotherapy compared to post-operative therapy in the treatment of locally advanced breast cancer. Cancer Invest 16:80–86
Semiglazov VF, Topuzov EE, Bavli JL, Moiseyenko VM, Ivanova OA, Seleznev IK, Orlov AA, Barash NY, Golubeva OM, Chepic OF (1994) Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage Iib–IIIa breast cancer. Ann Oncol 5:591–595
Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, Dilhuydy JM, Bonichon F (1999) Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol 10:47–52
Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, Coleman RE, Perren T, Gallagher CJ, Quigley M, Crown J, Jones AL, Highley M, Leonard RC, Mansi JL (2005) Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an anglo–celtic cooperative oncology group study. J Clin Oncol 23:2988–2995
Nabholtz JM, Thuerlimann B, Bezwoda WR, Melnychuk D, Deschenes L, Douma J, Vandenberg TA, Rapoport B, Rosso R, Trillet-Lenoir V, Drbal J, Aapro MS, Alaki M, Murawsky M, Riva A (1997) Docetaxel versus mitomycin plus vinblastine in anthracycline-resistant metastatic breast cancer. Oncology (Williston Park) 11:25–30
Schwartz GF, Cantor RI, Biermann WA (1987) Neoadjuvant chemotherapy before definitive treatment for stage III carcinoma of the breast. Arch Surg 122:1430–1434
Pelissier P DS, Mathieu M-C et al (2002) Intensified anthracycline does not improve clinical and pathological responses to neoadjuvant FEC for operable breast cancer: results of a multicenter randomised trial. Proc Am Soc Clin Oncol 21:649 (abstr 254)
Misset JL, Dieras V, Gruia G, Bourgeois H, Cvitkovic E, Kalla S, Bozec L, Beuzeboc P, Jasmin C, Aussel JP, Riva A, Azli N, Pouillart P (1999) Dose-finding study of docetaxel and doxorubicin in first-line treatment of patients with metastatic breast cancer. Ann Oncol 10:553–560
Palmeri S, Leonardi V, Tamburo De Bella M, Morabito A, Vaglica M, Accurso V, Ferrau F, Failla G, Agostara B, Massidda B, Valenza R, Fanelli M,Gasparini G (2002) Doxorubicin-docetaxel sequential schedule: results of front-line treatment in advanced breast cancer. Oncology 63:205–212
Aihara T, Takatsuka Y, Itoh K, Sasaki Y, Katsumata N, Watanabe T, Noguchi S, Horikoshi N, Tabei T, Sonoo H, Hiraki S, Inaji H (2003) Phase II study of concurrent administration of doxorubicin and docetaxel as first-line chemotherapy for metastatic breast cancer. Oncology 64:124–130
Ganem G, Tubiana-Hulin M, Fumoleau P, Combe M, Misset JL, Vannetzel JM, Bachelot T, De Ybarlucea LR, Lotz V, Bendahmane B,Dieras V (2003) Phase II trial combining docetaxel and doxorubicin as neoadjuvant chemotherapy in patients with operable breast cancer. Ann Oncol 14:1623–1628
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
EBCTCG (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Early breast cancer trialists’ collaborative group. Lancet 351:1451–1467
Oh YL, Choi JS, Song SY, Ko YH, Han BK, Nam SJ, Yang JH (2001) Expression of p21Waf1, p27Kip1 and cyclin D1 proteins in breast ductal carcinoma in situ: relation with clinicopathologic characteristics and with p53 expression and estrogen receptor status. Pathol Int 51:94–99
Allred DC, Bustamante MA, Daniel CO, Gaskill HV, Cruz AB Jr (1990) Immunocytochemical analysis of estrogen receptors in human breast carcinomas. Evaluation of 130 cases and review of the literature regarding concordance with biochemical assay and clinical relevance. Arch Surg 125:107–113
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, Buzdar AU, Garbay JR, Spielmann M, Mathieu MC, Symmans WF, Wagner P, Atallah D, Valero V, Berry DA, Hortobagyi GN (2005) Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol 23:8331–8339
Chang J, Powles TJ, Allred DC, Ashley SE, Clark GM, Makris A, Assersohn L, Gregory RK, Osborne CK, Dowsett M (1999) Biologic markers as predictors of clinical outcome from systemic therapy for primary operable breast cancer. J Clin Oncol 17:3058–3063
Ellis P, Smith I, Ashley S, Walsh G, Ebbs S, Baum M, Sacks N,McKinna J (1998) Clinical prognostic and predictive factors for primary chemotherapy in operable breast cancer. J Clin Oncol 16:107–114
Miller WR, Dixon JM, Macfarlane L, Cameron D, Anderson TJ (2003) Pathological features of breast cancer response following neoadjuvant treatment with either letrozole or tamoxifen. Eur J Cancer 39:462–468
Anderson TJ, Dixon JM, Stuart M, Sahmoud T, Miller WR (2002) Effect of neoadjuvant treatment with anastrozole on tumour histology in post-menopausal women with large operable breast cancer. Br J Cancer 87:334–338
Miller WR, White S, Dixon JM, Murray J, Renshaw L, Anderson TJ (2006) Proliferation, steroid receptors and clinical/pathological response in breast cancer treated with letrozole. Br J Cancer 94:1051–1056
Tao Y, Klause A, Vickers A, Bae K, Ellis M (2005) Clinical and biomarker endpoint analysis in neoadjuvant endocrine therapy trials. J Steroid Biochem Mol Biol 95:91–95
Ding SL, Sheu LF, Yu JC, Yang TL, Chen B, Leu FJ, Shen CY (2004) Expression of estrogen receptor-alpha and Ki67 in relation to pathological and molecular features in early-onset infiltrating ductal carcinoma. J Biomed Sci 11:911–919
Russo J, Ao X, Grill C, Russo IH (1999) Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat 53:217–227
Clarke RB, Howell A, Potten CS, Anderson E (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57:4987–4991
von Minckwitz G, Costa SD, Raab G, Blohmer JU, Eidtmann H, Hilfrich J, Merkle E, Jackisch C, Gademann G, Tulusan AH, Eiermann W, Graf E, Kaufmann M (2001) Dose-dense doxorubicin, docetaxel, and granulocyte colony-stimulating factor support with or without tamoxifen as pre-operative therapy in patients with operable carcinoma of the breast: a randomized, controlled, open phase IIb study. J Clin Oncol 19:3506–3515
Miller KD, McCaskill-Stevens W, Sisk J, Loesch DM, Monaco F, Seshadri R, Sledge GW Jr (1999) Combination versus sequential doxorubicin and docetaxel as primary chemotherapy for breast cancer: a randomized pilot trial of the Hoosier Oncology Group. J Clin Oncol 17:3033–3037
Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, Ah-See AK, Eremin O, Walker LG, Sarkar TK, Eggleton SP, Ogston KN (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
von Minckwitz G, Raab G, Caputo A, Schutte M, Hilfrich J, Blohmer JU, Gerber B, Costa SD, Merkle E, Eidtmann H, Lampe D, Jackisch C, du Bois A, Kaufmann M (2005) Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 23:2676–2685
Reitsamer R, Peintinger F, Prokop E, Hitzl W (2005) Pathological complete response rates comparing three versus six cycles of epidoxorubicin and docetaxel in the neoadjuvant setting of patients with stage II and III breast cancer. Anticancer Drugs 16:867–870
Acknowledgments
This study was supported by a grant of the Korean Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (0412-CR01-0704-001).
Conflict of Interest
Authors claim no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Im, Y.H., Lee, S.H. et al. Evaluation of ER and Ki-67 proliferation index as prognostic factors for survival following neoadjuvant chemotherapy with doxorubicin/docetaxel for locally advanced breast cancer. Cancer Chemother Pharmacol 61, 569–577 (2008). https://doi.org/10.1007/s00280-007-0506-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0506-8