Abstract
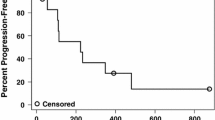
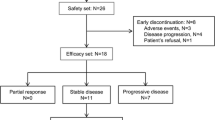
Purpose: This phase II study evaluated the combination of semaxanib, a small molecule tyrosine kinase inhibitor of vascular endothelial growth factor (VEGF) receptor-2, and thalidomide in patients with metastatic melanoma to assess the efficacy, tolerability, pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of the combination. Patients and methods: Patients with metastatic melanoma, who had failed at least one prior biologic and/or chemotherapeutic regimen, were treated with escalating doses of thalidomide combined with a fixed dose of semaxanib. Results: Twelve patients were enrolled and received 44 courses of semaxanib at the fixed dose of 145 mg/m2 intravenously twice-weekly in combination with thalidomide, commencing at 200 mg daily with intrapatient dose escalation as tolerated. Treatment with semaxanib was initiated 1 day before thalidomide in the first course, permitting the assessment of the PKs of semaxanib alone (course 1) and in combination with thalidomide (course 2). The principal toxicities included deep venous thrombosis, headache, and lower extremity edema. Of ten patients evaluable for response, one complete response lasting 20 months and one partial response lasting 12 months were observed. Additionally, four patients had stable disease lasting from 2 to 10 months. The PKs of semaxanib were characterized by drug exposure parameters comparable to those observed in single-agent phase II studies, indicating the absence of major drug–drug interactions. Maximum semaximib plasma concentration values were 1.2–3.8 μg/ml in course 1 and 1.1–3.9 μg/ml in course 2. The mean terminal half-life was 1.3 ( ± 0.31) h. Biological studies revealed increasing serum VEGF concentrations following treatment in patients remaining on study for more than 4 months. Conclusion: The combination of semaxanib and thalidomide was feasible and demonstrated anti-tumor activity in patients with metastatic melanoma who had failed prior therapy. Further evaluations of therapeutic strategies that target multiple angiogenesis pathways may be warranted in patients with advanced melanoma and other malignancies.


Similar content being viewed by others
References
Jemal A, Murray T, Samuels A, et al (2003) Cancer statistics 2003. CA Cancer J Clin 53:5–26
Gloeckler Ries LA, Reichman ME, Lewis DR, et al (2003) Cancer survival and incidence from the surveillance, epidemiology, and end results (SEER) program. Oncologist 8:541–552
Anderson C, Buzaid AC, Legha SS (1995) Systemic treatment for advanced cutaneous melanoma. Oncology 9:1149–1158
Einzig AI, Hochster H, Wiernik PH, et al (1991) A phase II study of taxol in patients with malignant melanoma. Invest New Drugs 9:59–64
Bedikian AY, Weiss GR, Legha SS, et al (1995) A phase II trial of docetaxel in patients with advanced cutaneous melanoma untreated with chemotherapy. J Clin Oncol 13:2895–2899
Gorski DH, Leal AD, Goydos JS (2003) Differential expression of vascular endothelial growth factor A isoforms at different stages of melanoma progression. J Am Coll Surg 197:408–418
Erhard H, Rietveld FJ, van Altena MC, et al (1997) Transition of horizontal to vertical growth phase melanoma is accompanied by induction of vascular endothelial growth factor expression and angiogenesis. Melanoma Res 7(Suppl. 2):S19–S26
Ugurel S, Rappl G, Tigen W, Reinhold U (2001) Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol 19:577–583
Prewett M, Huber J, Li Y, et al (1999) Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth in several mouse and human tumors. Cancer Res 59:5209–5218
Siemeister G, Schirner M, Weindel K, et al (1999) Two independent mechanisms essential for tumor angiogenesis: inhibition of human melanoma xenograft growth by interfering with either the vascular endothelial growth factor receptor pathway or the Tie-2 pathway. Cancer Res 59:3185–3191
D’Amato RJ, Loughnan MS, Flynn E, Folkman J (1994) Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 91:4082–4085
Ng SSW, Brown M, Figg WD (2002) Thalidomide, an antiangiogenic agent with clinical activity in cancer. Biomed Pharmacother 56:194–199
Franks ME, Macpherson GR, Figg WD (2004) Thalidomide. Lancet 363:1802–1811
Keifer JA, Guttridge DC, Ashburner BP, et al (2001) Inhibition of NF-kappa B activity by thalidomide through suppression of I kappa B kinase activity. J Biol Chem 276:22383–22387
Geitz H, Handt S, Zwingerberger K (1996) Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology 31:213–221
Shinghal S, Metha J, Desikan R, et al (1999) Anti-tumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 341:1565–1571
Weber D, Rankin K, Gavino M, et al (2003) Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol 21:16–19
Barlogie B, Desikan R, Eddlemon P, et al (2001) Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase II study of 169 patients. Blood 15:492–494
Eisen T, Boshoff C, Mak I, et al (2000) Continuous low dose thalidomide: a phase II study I advanced melanoma, renal cell, ovarian and breast cancer. Br J Cancer 82:812–817
Raza A, Meyer P, Dutt D, et al (2001) Thalidomide produces transfusion independence in long-standing refractory anemias of patients with myelodysplastic syndromes. Blood 98:958–965
Mesa RA, Steensma DP, Pardani A, et al (2003) A phase II trial of combination low dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasia. Blood 101:2534–2541
Pawlak WZ, Legha SS (2004) Phase II study of thalidomide in patients with metastatic melanoma. Melanoma Res 14:57–62
Hwu WJ, Krown SE, Menell JH, et al (2003) Phase II study of temozolomide plus thalidomide for the treatment of metastatic melanoma. J Clin Oncol 21:3351–3356
Hwu W-J, Krown SE, Panageas KS, et al (2002) Temozolomide plus thalidomide in patients with advanced melanoma: results of a dose-finding trial. J Clin Oncol 20:2610–2615
Danson S, Lorigan P, Arance A, et al (2003) Randomized phase II study of temozolomide given every 8 hours or daily with either interferon alfa-2b or thalidomide in metastatic malignant melanoma. J Clin Oncol 21:2551–2557
Eisen T, Boshoff MM, Vaughan MM, et al (1998) Anti-angiogenic treatment of metastatic melanoma, renal cell, ovarian and breast cancers with thalidomide: a phase II study. Proc Am Soc Clin Oncol 17, abstract 1699a
Pavlick AC, Oratz R, Bailes A, et al (2002) A phase II trial of DTIC with thalidomide in metastatic melanoma. Proc Am Soc Clin Oncol, abstract 1393
Solti M, Mastrangelo MJ, Berd D, et al (2003) Phase II study of low dose thalidomide and interferon alfa 2-b (IFN) in patients with metastatic melanoma—preliminary results. Proc Am Soc Clin Oncol 22:725, abstract 2914
Fong TA, Shawver LK, Sun L, et al (1999) SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59:99–106
Huss W, Barrios R, Greenberg N, et al (2003) SU5416 selectively impairs angiogenesis to induce prostate cancer-specific apoptosis. Mol Cancer Ther 2:611–616
SU5416 and thalidomide phase II in patients with metastatic melanoma—investigator brochure
Mendel DB, Laird AD, Smolich BD, et al (2000) Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des 15:29–41
Mendel DB, Schreck RE, West DC, et al (2000) The angiogenesis inhibitor SU5416 has long lasting effects on VEGF receptor phosphorylation and function. Clin Cancer Res 6:4848–4858
Stopeck A, Sheldon M, Vahedian M, et al (2002) Results of a phase I dose-escalating study of the antiangiogenic agent SU5416, in patients with advanced malignancies. Clin Cancer Res 8:2798–2805
Peterson AC, Swinger S, Stadler WM, et al (2004) Phase II study of the FLk-1 tyrosine kinase inhibitor SU5416 in advanced melanoma. Clin Cancer Res 10:4048–4054
Simon R (1989) Optimal two-stage design for phase II clinical trials. Control Clin Trials 10:1–10
Kuenen BC, Rosen L, Smit EF, et al (2002) Dose-finding and pharmacokinetic study of cisplatin, gemcitabine and SU5416 in patients with solid tumors. J Clin Oncol 6:1657–1667
Gibaldi M, Perrier D (1982) Pharmacokinetics. Marcel Dekker, New York
Folkman J (1972) Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg 175:406–416
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Bergers G, Benjamin LA (2003) Tumorigenesis and angiogenic switch. Nature 3:401–410
Lee J, Chow N, Want S, et al (2000) Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer 36:748–753
Takahashi Y, Kitadai Y, Bucana CD, et al (1995) Expression of vascular endothelial growth factor receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 55:3964–3968
Maeda K, Chung YS, Ogawa Y, et al (1996) Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 77:858–863
Takahashi Y, Cleary KR, Mai M, et al (1996) Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal type gastric cancer. Clin Cancer Res 2:1679–1684
Fujimoto K, Hosotani R, Wada M, et al (1998) Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer 34:1439–1447
Ikeda N, Adachi M, Taki T, et al (1999) Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer 79:1553–1563
Berns EM, Klijn JG, Look MP, et al (2003) Combined vascular endothelial growth factor and TP53 status predicts poor response to tamoxifen therapy in estrogen receptor positive advanced breast cancer. Clin Cancer Res 9:1253–1258
Manders P, Beex LV, Tjan-Heijnen VC, et al (2002) The prognostic value of vascular endothelial growth factor in 574 node-negative breast cancer patients who did not receive adjuvant systemic therapy. Br J Cancer 87:772–778
George DJ, Halabi S, Shepard TF, et al (2001) Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res 7:1932–1936
Lee M Elllis (2004) The biology of VEGF and tumor angiogenesis. Horiz Cancer Ther 5(2):4–10
Hurwitz H, Fehrenbacher L, Cartwright T, et al (2003) Bevacizumab (a monoclonal antibody to vascular endothelial growth factor) prolongs survival in first line colorectal cancer (CRC): results of a phase III trial of bevacizumab in combination with bolus IFL (irinotecan, 5-fluorouracil, leucovorin) as first line therapy in subjects with metastatic colorectal cancer (CRC). Proc Am Soc Clin Oncol, abstract 3646
Abdollahi A, Lipson KE, Sckell A, et al (2003) Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res 63(24):8890–8898
Acknowledgments
We thank Dr. Jinee Rizzo for support of the PK assays and analysis. This work was supported by the NIH Grant UO1-CA69853.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 39th meeting of American Society of Clinical Oncology Chicago, IL, May 2003.
Rights and permissions
About this article
Cite this article
Mita, M.M., Rowinsky, E.K., Forero, L. et al. A phase II, pharmacokinetic, and biologic study of semaxanib and thalidomide in patients with metastatic melanoma. Cancer Chemother Pharmacol 59, 165–174 (2007). https://doi.org/10.1007/s00280-006-0255-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0255-0