Abstract
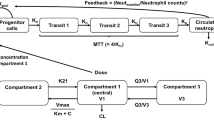
Purpose: The objectives of these analyses were to (1) develop a semimechanistic-physiologic population pharmacokinetic/pharmacodynamic (PK/PD) model to describe neutropenic response to pemetrexed and to (2) identify influential covariates with respect to pharmacodynamic response. Patients and methods: Data from 279 patients who received 1,136 treatment cycles without folic acid or vitamin B12 supplementation during participation in one of eight phase II cancer trials were available for analysis. Starting doses were 500 or 600 mg pemetrexed per m2 body surface area (BSA), administered as 10-min intravenous infusions every 21 days (1 cycle). The primary analyses included 105 patients (279 cycles) for which selected covariates—including vitamin deficiency marker data (i.e., homocysteine, cystathionine, methylmalonic acid, and methylcitrate [I, II, and total] plasma concentrations)—were available. Classical statistical multivariate regression analyses and a semimechanistic-physiologic population PK/PD model were used to evaluate neutropenic response to single-agent pemetrexed administration. Results: The timecourse of neutropenia following single-agent pemetrexed administration was adequately described by a semimechanistic-physiologic model. Population estimates for system-based model parameters (i.e., baseline neutrophil count, mean transit time, and the feedback parameter), which mathematically represent current understanding of the process and physiology of hematopoiesis, were consistent with previously reported values. The population PK/PD model included homocysteine, cystathionine, albumin, total protein, and BSA as covariates relative to neutropenic response. Conclusion: These results support the programmatic decision to introduce folic acid and vitamin B12 supplementation during pemetrexed clinical development as a means of normalizing patient homocysteine levels, thereby managing the risk of severe neutropenia secondary to pemetrexed administration. The current results also suggest that the addition of vitamin B6 supplementation to normalize patient cystathionine levels may further decrease the incidence of grade 4 neutropenia following pemetrexed administration. The results also suggest the use of folic acid as a means of lessening hematologic toxicity following administration of cytotoxic agents other than antifolates.









Similar content being viewed by others
References
Adjei AA (2003) Pemetrexed (Alimta): a novel multitargeted antifolate agent. Expert Rev Anticancer Ther 3:145–156
Alati T, Shih C, Pohland RC, Lantz FJ, Grindey GB (1992) Evaluation of the mechanism(s) of inhibition of the toxicity, but not the antitumor activity of lomotrexol (DDATHF) by folic acid. Proc Am Assoc Cancer Res 33:407
Alimta Drug Approval Package, Medical Review. Food and Drug Administration Web site. Available at: http://www.fda.gov/cder/foi/nda/2004/21-462.pdf_Alimta_Medr_P6.pdf. Page 257. Accessed September 20, 2004
Allen RH, Stabler SP, Savage DG, Lindenbaum J (1993) Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J 7:1344–1353
Bajetta E, Celio L, Buzzoni R et al (2003) Phase II study of pemetrexed disodium (Alimta) administered with oral folic acid in patients with advanced gastric cancer. Ann Oncol 14:1543–1548
Beal SL, Sheiner LB (1982) Estimating population kinetics. Crit Rev Biomed Eng 8:195–222
Beal SL, Sheiner LB (1988) Heterskedastic nonlinear regression. Technometrics 30:327–338
Beal SL, Sheiner LB (1992) NONMEM user’s guide NONMEM project group. University of California, San Francisco
Branda RF, Nigels E, Lafayette AR, Hacker M (1998) Nutritional folate status influences the efficacy and toxicity of chemotherapy in rats. Blood 92:2471–2476
Branda RF, Chen Z, Brooks EM, Naud SJ, Trainer TD, McCormack JJ (2002) Diet modulates the toxicity of cancer chemotherapy in rats. J Lab Clin Med 140:358–368
Branda RF, Naud SJ, Brooks EM, Chen Z, Muss H (2004) Effect of vitamin B12, folate, and dietary supplements on breast carcinoma chemotherapy-induced mucositis and neutropenia. Cancer 101:1058–1064
Carmel R, Jacobsen DW (2001) (eds) Homocysteine in health and disease. Cambridge University Press, New York
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Cripps C, Burnell M, Jolivet J et al (1999) Phase II study of first-line LY231514 (multitargeted antifolate) in patients with locally advanced or metastatic colorectal cancer: an NCIC Clinical Trials Group study. Ann Oncol 10:1175–1179
Ette EI (1997) Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 37:486–495
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO (2002) Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20:4713–4721
Goedhals L, van Wijk AL (1998) MTA (LY231514) in advanced carcinoma of the cervix. Ann Oncol 9(suppl 4):70
Grindey GB, Alati T, Shih C (1991) Reversal of the toxicity but not the antitumor activity of lometrexol by folic acid. Proc Am Assoc Cancer Res 32:324
Grindey GB, Shih C, Barnett CJ et al (1992) LY231514, a novel pyrrolopyrimidine antifolate that inhibits thymidylate synthase (TS). Proc Am Assoc Cancer Res 33:411
Hanauske AR, Chen V, Paoletti P, Niyikiza C (2001) Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist 6:363–373
Hanna N, Shepherd FA, Fossella FV et al (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597
Homocysteine Lowering Trialists’ Collaboration (1998) Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ 316:894–898
Laohavinij S, Wedge SR, Lind MJ et al (1996) A phase I clinical study of the antipurine antifolate lomotrexol (DDATHF) given with oral folic acid. Invest New Drugs 14:325–335
Latz JE, Chaudhary A, Ghosh A, Johnson RD (2006) Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol 57. DOI 10.1007/s00280-005-0036-1
Latz JE, Rusthoven JJ, Karlsson MO, Ghosh A, Johnson RD (2006) Clinical application of a semimechanistic-physiologic population PK/PD model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol 57. DOI 10.1007/s00280-005-0035-2
Maas H (2001) Development of a semi-physiological population PK/PD model of hematological toxicity: evaluation of across-drug consistency. MSc Thesis. Uppsala University. Uppsala, Sweden
Mackiewicz A, Speroff T, Ganapathi MK, Kushner I (1991) Effects of cytokine combinations on acute phase protein production in two human hepatoma cell lines. J Immunol 146:3032–3037
Maes M, Stevens W, Scharpe S et al (1994) Seasonal variation in peripheral blood leukocyte subsets and in serum interleukin-6 and soluble interleukin-2 and −6 receptor concentrations in normal volunteers. Experientia 50:821–829
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm 20:511–528
McKenzie SB (1996) Textbook of hematology. Williams and Wilkins, Baltimore, pp 22–29, pp 58–67
Mentre F, Ebelin ME (1997) Validation of population pharmacokinetic/pharmacodynamic analyses: review of proposed approaches. In: Balant LP, Aarons L (eds) The population approach: measuring and managing variability in response, concentration and dose. Commission of the European Communities, Brussels, pp 147–160
Miller KD, Picus J, Blanke C et al (2000) Phase II study of the multitargeted antifolate LY231514 (ALIMTA, MTA, pemetrexed disodium) in patients with advanced pancreatic cancer. Ann Oncol 11:101–103
Moran RG (1999) Roles of folylpoly-gamma-glutamate synthetase in therapeutics with tetrahydrofolate antimetabolites: an overview. Semin Oncol 26(2 suppl 6):24–32
Niyikiza C, Baker SD, Seitz DE et al (2002) Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther 1:545–552
Niyikiza C, Hanauske AR, Rusthoven JJ et al (2002) Pemetrexed safety and dosing strategy. Semin Oncol 29(6 Suppl 18):24–29
Odamaki M, Kato A, Kumagai H, Hishida A (2004) Counter-regulatory effects of procalcitonin and indoxyl sulphate on net albumin secretion by cultured rat hepatocytes. Nephrol Dial Transplant 19:797–804
O’Dwyer PJ, Nelson K, Thornton DE (1999) Overview of phase II trials of MTA in solid tumors. Semin Oncol 26:99–104
Paz-Ares L, Tabernero J, Moyano A et al (1999) Significant activity of the multitargeted antifolate MTA (LY231514) in advanced transitional cell carcinoma (TCC) of the bladder: results of a phase II trial. Eur J Cancer 35(suppl 2):S81
Pivot X, Raymond E, Laguerre B et al (2001) Pemetrexed disodium in recurrent locally advanced or metastatic squamous cell carcinoma of the head and neck. Br J Cancer 85:649–655
Rinaldi DA, Kuhn JG, Burris HA et al (1999) A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol 44:372–380
Rusthoven JJ, Eisenhauer E, Butts C et al (1999) Multitargeted antifolate LY231514 as first-line chemotherapy for patients with advanced non-small cell lung cancer: a phase II study National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 17:1194–1199
Sandström M, Lindman H, Nygren P, Lidbrink E, Bergh J, Karlsson MO (2005) Model describing the relationship between pharmacokinetics and hematologic toxicity of the epirubicin-docetaxel regimen in breast cancer patients. J Clin Oncol 23:413–421
SAS Institute Inc (1997) SAS/STAT software: changes and enhancements through release 6.12. Cary, NC: SAS Institute Inc
Scagliotti GV, Shin DM, Kindler HL et al (2003) Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 21:1556–1561
Shargel L, Yu ABC (1985) Applied biopharmaceutics, 2nd edn. Appleton-Century-Crofts, Norwalk, pp 310–312
Sheiner LB (1986) Analysis of pharmacokinetic data using parametric models. III. Hypothesis tests and confidence intervals. J Pharmacokinet Biopharm 14:539–555
Sheiner LB, Steimer JL (2000) Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol 40:67–95
Shih C, Gosset L, Gates S et al (1996) LY231514 and its polyglutamates exhibit potent inhibition against both human dihydrofolate reductase (DHFR) and thymidylate synthase (TS): multiple folate enzyme inhibition. Ann Oncol 7(Suppl 1):85
Spielmann M, Martin M, Namer M, duBois A, Unger C, Dodwell DJ (2001) Activity of pemetrexed (ALIMTA, multitargeted antifolate, LY231514) in metastatic breast cancer patients previously treated with an anthracycline and a taxane: an interim analysis. Clin Breast Cancer 2:47–51
Stabler SP, Lindenbaum J, Savage DG, Allen RH (1993) Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood 81:3404–3413
Sun H, Fadiran EO, Jones CD et al (1999) Population pharmacokinetics. A regulatory perspective. Clin Pharmacokinet 37: 41–58
Suwa T, Hogg JC, English D, Van Eeden SF (2000) Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol 279:H2954–H2960
Vogelzang NJ, Rusthoven JJ, Symanowski J et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
Wedge SR, Laohavinij S, Taylor GA, Boddy A, Calvert AH, Newell DR (1995) Clincial pharmacokinetics of the antipurine antifolate (6R)-5,10- dideaza-5,6,7,8-tetrahydrofolic acid (Lomotrexol) administered with an oral folic acid supplement. Clin Cancer Res 1:1479–1486
Yoshioka M, Mori Y, Miyazaki S, Miyamoto T, Yokomizo Y, Nakajima Y (1999) Biological functions of recombinant bovine interleukin 6 expressed in a baculovirus system. Cytokine 11:863–868
Acknowledgements
The authors wish to thank the following individuals for their assistance with the conduct of these analyses and preparation of this manuscript: Dinesh Dealwis, Lena Friberg, and S Thomas Forgue for their insightful scientific comments; Mary Brandes Dugan and Pete Fairfield for their scientific writing and editorial support; and, David B. Radtke for operations and project management support.
Author information
Authors and Affiliations
Corresponding author
Additional information
James J Rusthoven, Robert D Johnson were employees of Eli Lilly and Company, Indianapolis, USA, at the time this work was completed
Rights and permissions
About this article
Cite this article
Latz, J.E., Karlsson, M.O., Rusthoven, J.J. et al. A semimechanistic-physiologic population pharmacokinetic/pharmacodynamic model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol 57, 412–426 (2006). https://doi.org/10.1007/s00280-005-0077-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0077-5