Abstract
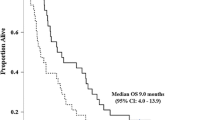
Purpose: To determine the maximum tolerated dose and dose-limiting toxicity (DLT) of the novel anticancer agent, motexafin gadolinium (MGd), administered concurrently with radiation therapy (RT) in patients with locally advanced pancreatic or biliary tumors. The pharmacokinetics of MGd were also evaluated. Methods: Cohorts of three to six patients were treated with escalating doses of MGd, administered three times per week for a total of 16 doses concurrent with RT. The dose of RT was fixed at 5,040 cGy, and given in 28 fractions, from Monday to Friday of every week. Plasma MGd concentrations were measured by high performance liquid chromatography. Results: Eight patients were treated at dose level 1 (2.9 mg/kg), with one DLT (grade 3 fever). Three patients were treated at dose level 2 (3.6 mg/kg), and two DLTs were noted. One DLT was grade 3 nausea and vomiting (N/V), and the other was grade 3 skin toxicity. The most common toxicity was N/V. There were no objective responses. The median survival was 6 months. The MGd plasma concentration versus time profile in each patient was best fit by a two-compartment, open, linear model. There was minimal accumulation of MGd in plasma with the three-times/week dosing schedule. Simulation of the time course of MGd in the peripheral compartment indicated that maximal MGd concentrations of 1–2 μmol/kg occurred between 4 and 6 h after MGd infusion. Conclusion: Dose level 1 (2.9 mg/kg of MGd) is the recommended dose for combination with (RT) in phase II studies for locally advanced pancreatic and biliary cancers. Patient tolerance might be improved by modification of the RT schedule and antiemetic prophylaxis.





Similar content being viewed by others
References
Moertel CG, Frytak S, Hahn RG, O’Connell MJ et al (1981) Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 48:1705–1710
Anonymous (1988) Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst 80:751–755
Crane CH, Abbruzzese JL, Evans DB et al (2002) Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys 52:1293–1302
Muler J, McGinn C, Normolle D, Lawrence T, Brown D, Hejna G, Zalupski M (2004) Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol 22:238–243
Safran H, Dipetrillo T, Iannitti D et al (2002) Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: a Phase I trial. Int J Radiat Oncol Biol Phys 54:137–141
McGinn C, Zalupski M, Shureiqi I et al (2001) Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 19:4202–4208
Rich T, Harris J, Abrams R et al (2004) Phase II study of external irradiation and weekly paclitaxel for nonmetastatic, unresectable pancreatic cancer: RTOG-98-12. J Clin Oncol 27:51–56
Erickson BA, Nag S (1998) Biliary tree malignancies. J Surg Oncol 67:203–210
Miller RA, Woodburn K, Fan Q et al (1999) In vivo animal studies with gadolinium(III) texaphyrin as a radiation enhancer. Int J Radiat Oncol Biol Phys 45:981–989
Sessler JL, Miller RA (2000) Texaphyrins: new drugs with diverse clinical applications in radiation and photodynamic therapy. Biochem Pharmacol 59:733–739
Magda D, Lepp C, Gerasimchuk N et al (2001) Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen species. Int J Radiat Oncol Biol Phys 51:1025–1036
Young SW, Quing F, Harriman A et al (1996) Gadolinium(III) texaphyrin: a tumor selective radiation sensitizer that is detectable by MRI. Proc Natl Acad Sci USA 93:6610–6615
Carde P, Timmerman R, Mehta MP et al (2001) Multicenter phase Ib/II trial of the radiation enhancer motexafin gadolinium in patients with brain metastases. J Clin Oncol 19:2074–2083
Mehta MP, Shapiro WR, Glantz MJ (2002) Lead-in phase to randomized trial of motexafin gadolinium and whole-brain radiation for patients with brain metastases: centralized assessment of magnetic resonance imaging, neurocognitive, and neurologic end points. J Clin Oncol 20:3445–3453
Rosenthal DI, Nurenberg P, Becerra CR (1999) A phase I single-dose trial of gadolinium texaphyrin (Gd-Tex), a tumor selective radiation sensitizer detectable by magnetic resonance imaging. Clin Cancer Res 5:739–745
Mehta MP, Rodrigus P, Terhaard CHJ et al (2003) Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 21:2529–2536
Meyers CA, Smith JA, Bezjak A et al (2004) Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol 22:157–165
Pharmacyclics Corporation (2004) Investigators brochure for Motexfin gadolinium
Miller AB, Hoogstraten B, Staquet M et al (1981) Reporting results of cancer treatment. Cancer 47:207–214
Parise RA, Miles DR, Egorin MJ (2000) Sensitive high-performance liquid chromatographic assay for motexafin gadolinium and motexafin lutetium in human plasma. J Chromatogr B Biomed Sci Appl 749:145–152
D’Argenio DZ, Schumitzky A (1979) A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed 9:115–134
Akaike H (1979) A Bayesian extension of the minimal AIC procedures of autoregressive model fitting. Biometrika 66:237–242
Miles DR, Smith JA, Phan SC et al (2005) Population pharmacokinetics of motexafin gadolinium in adults with brain metastases or glioblastoma multiforme. J Clin Pharm 45:299–312
Miller RA, Fan Q, Lee I et al (2002) Motexafin gadolinium (MGd) increases tumor response to chemotherapy in Lewis lung cancer (LLC) animal model. Proc Am Soc Clin Oncol 21: (Abstract#466)
Magda D, Lepp C, Fan Q et al (2003) The redox mediator motexafin gadolinium (MGd) enhances the activity of several chemotherapy drugs. Proc Am Soc Clin Oncol 23:229 (Abstract#917)
Ramnath N, Chatta G, Egorin MJ, Phan S, Creaven PJ (2004) A phase 1 trial of motexafin gadolinium and docetaxel for advanced solid tumors. Proc Am Soc Clin Oncol 22: (Abstract#3171)
Thomas JP, Ramanathan RK, Wilding G et al (2003) A phase I study of motexafin gadolinium (MGd) in combination with doxorubicin (Dox). Proc Am Soc Clin Oncol 22:227 (Abstract#909)
Evens AM, Lecane P, Magda D et al (2005) Motexafin gadolinium generates reactive oxygen species and induces apoptosis in sensitive and highly resistant multiple myeloma cells. Blood 105:1265–1273
Acknowledgments
We thank Ms. Alicia Depastino and Mr. Jeremy Hedges for excellent secretarial support and the UPCI Hematology/Oncology Writing Group for constructive criticisms regarding this manuscript. This study was sponsored by the Cancer Therapy and Evaluation Program of the National Cancer Institute, Bethesda, MD, USA. Presented, in part, at the 37th Annual Meeting of the American Society of Clinical Oncology, San Francisco, CA, 2001. Supported, in part, by grants UO1-CA69855, P30CA47904, NIH/NCCR/GCRC#5M01 RR 00056 to the University of Pittsburgh Cancer Institute and Medical Center. U01-CA069852-11 to University of Chicago Medical Center, NIH grants K24 CA84081 and P30CA23108 to Dartmouth College and U01-CA62491/CA/NCI to the University of Wisconsin Comprehensive Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramanathan, R.K., Fakih, M., Mani, S. et al. Phase I and pharmacokinetic study of the novel redox-active agent, motexafin gadolinium, with concurrent radiation therapy in patients with locally advanced pancreatic or biliary cancers. Cancer Chemother Pharmacol 57, 465–474 (2006). https://doi.org/10.1007/s00280-005-0071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0071-y