Abstract
Purpose
Perillyl alcohol (POH) has been shown to have both chemopreventative and chemotherapeutic activities in preclinical studies. The underlying mechanism(s) of action of POH have yet to be delineated but may involve effects on the transforming growth factor β (TGFβ) and/or the Ras signaling pathways. A phase I study of POH for 14 days out of every 28 days in subjects with advanced malignancies was performed to evaluate dose escalation, toxicity, pharmacokinetics, and effects on TGFβ and Ras.
Methods
POH was administered orally (500 mg capsules containing 250 mg POH) to 20 patients four times a day on a continuous basis for 14 days followed by a 14-day rest period, for up to three courses. The starting dose was 1200 mg/m2 per dose. A minimum of three patients were treated and evaluated at each escalating POH dose. Pharmacokinetic analysis was performed on days 1 and 14 of course 1 and day 1 of selected later courses. Plasma TGFβ levels were measured on days 1 and 14. Peripheral blood lymphocyte (PBLs) Ras levels were assayed on days 1 and 2 of the first course.
Results
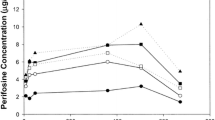
The 20 patients, of whom 15 were evaluable, received doses between 1200 and 2000 mg/m2 per dose for a total of 43 courses. The most common observed toxicities were nausea, gastrointestinal distress, and fatigue. Other toxicities included diarrhea or constipation, hypokalemia, and one incidence of acute pancreatitis. Due to these toxicities, four of the patients declined further treatment either during or after the second course. While POH was not detected in plasma, perillic acid (PA) and dihydroperillic acid (DHPA) were detected in plasma, and the peak levels at 2000 mg/m2 per dose were approximately 600 μM (PA) and 50 μM (DHPA). There was some evidence for linearity in the peak plasma levels and area under the concentration–time curve of the metabolites from the starting dose to the highest dose. Metabolite pharmacokinetics were not significantly affected by ingestion in the fed or fasting state, or repeated exposure to POH. No evidence for an effect of POH on plasma TGFβ or PBL Ras protein was observed. No objective responses were observed.
Conclusions
In adults with advanced malignancies, an interrupted administration schedule of POH did not reveal significant advantages over continuous dosing schedules.




Similar content being viewed by others
References
Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN (1999) Activation of the transforming growth factor β (beta) signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res 59:1917
Burke YD, Stark MJ, Roach SL, Sen SE, Crowell PL (1997) Inhibition of pancreatic cancer growth by the isoprenoids farnesyl and geraniol. Lipids 32:151
Crowell PL, Chang RR, Ren ZB, Elson CE, Gould MN (1991) Selective inhibition of isoprenylation of 21–26-kDa proteins by the anticarcinogen d-limonene and its metabolites. J Biol Chem 266:17679
Crowell PL, Ren Z, Lin S, Vedejs E, Gould M (1994) Structure-activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem Pharmacol 47:1404
Elegbede A, Elson CE, Tanner MA, Qureshi A, Gould MN (1986) Regression of rat mammary tumors following dietary d-limonene. J Natl Cancer Inst 76:323
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker, New York
Haag JD, Gould MN (1994) Mammary carcinoma regression induced by perillyl alcohol, a hydroxylated analog of limonene. Cancer Chemother Pharmacol 34:477
Haag JD, Lindstrom MJ, Gould MN (1992) Limonene-induced regression of mammary carcinomas. Cancer Res 52:4021
Hardcastle IR, Rowlands MG, Barber AM, Grimshaw RM, Mohan MK, Nutley BP, Jarman M (1999) Inhibition of protein prenylation by metabolites of limonene. Biochem Pharmacol 57:801
Hohl RJ, Lewis K (1995) Differential effects of monoterpenes and lovastatin on RAS processing. J Biol Chem 270:17508
Holstein SA, Hohl RJ (2003) Monoterpene regulation of Ras and Ras-related protein expression. J Lipid Res 44:1209
Hudes GR, Szarka CE, Adams A, Ranganathan S, McCauley RA, Weiner LM, Halberr T, Qian M, Gallo JM (2000) Phase I pharmacokinetic trial of perillyl alcohol (NSC 641066) in patients with refractory solid malignancies. Clin Cancer Res 6:3071
Jirtle RL, Haag JD, Ariazi EA, Gould MN (1993) Increased levels of mannose 6-phosphate/insulin-like growth factor II (M6P/IGF-II) receptor and transforming growth factor beta 1 (TGF-β1) levels during monoterpene-induced regression of mammary tumors. Cancer Res 53:3849
Kanai T, Hirohashi S, Noguchi M, Shimoyama Y, Shimosato Y, Noguchi S, Nishimura S, Abe O (1987) Monoclonal antibody highly sensitive for the detection of ras p21 in immunoblotting analysis. Jpn J Cancer Res 78:1314
Lluria-Prevatt M, Morreale J, Gregus J, Alberts DS, Kaper F, Giaccia A, Broome Powell M (2002) Effects of perillyl alcohol on melanoma in the TPras mouse model. Cancer Epidemiol Biomarkers Prev 11:573
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265
Murren JR, Pizzorno G, DiStasio SA, McKeon A, Peccerillo K, Gollerkari A, McMurray W, Burtness BA, Rutherford T, Li X, Ho PT, Sartorelli A (2002) Phase I study of perillyl alcohol in patients with refractory malignancies. Cancer Biol Ther 1:130
Phillips LR, Malspeis L, Supko JG (1995) Pharmacokinetics of active drug metabolites after oral administration of perillyl alcohol, an investigational antineoplastic agent, to the dog. Drug Metab Dispos 23:676
Reddy BS, Wang CX, Samaha H, Lubet R, Steele VE, Kelloff GJ, Rao CV (1997) Chemoprevention of colon carcinogenesis by dietary perillyl alcohol. Cancer Res 57:420
Ripple GH, Gould MN, Stewart JA, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Pomplun M, Wilding G, Bailey HH (1998) Phase I clinical trial of perillyl alcohol administered daily. Clin Cancer Res 4:1159
Ripple GH, Gould MN, Arzoomanian RZ, Alberti D, Feierabend C, Simon K, Binger K, Tutsch KD, Pomplun M, Wahamaki A, Marnocha R, Wilding G, Bailey HH (2000) Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clin Cancer Res 6:390
Shi W, Gould MN (2002) Induction of cytostasis in mammary carcinoma cells treated with the anticancer agent perillyl alcohol. Carcinogenesis 23:131
Vigushin DM, Poon G, Boddy A, Jarman M, Coombes RC (1998) Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer. Cancer Chemother Pharmacol 42:111
Wattenberg LW, Sparnins VL, Coccia JB (1989) Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res 49:2689
Acknowledgements
Supported by grants U01 CA62591 (UW), M01 RR03186 (UW), and M01 RR00059 (UI) from the National Institutes of Health and a grant (#97A028) from the American Institute for Cancer Research (R.J.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bailey, H.H., Wilding, G., Tutsch, K.D. et al. A phase I trial of perillyl alcohol administered four times daily for 14 days out of 28 days. Cancer Chemother Pharmacol 54, 368–376 (2004). https://doi.org/10.1007/s00280-004-0788-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0788-z