Abstract
Gastrointestinal mucositis could potentially compromise drug absorption due to functional loss of mucosa and other pathophysiological changes in the gastrointestinal microenvironment. Little is known about this effect on commonly used anti-infectives. This study aimed to explore the association between different stages of gastrointestinal mucositis, drug exposure, and gut microbiota. A prospective, observational pilot study was performed in HSCT patients aged ≥ 18 years receiving anti-infectives orally. Left-over blood samples and fecal swabs were collected from routine clinical care until 14 days after HSCT to analyze drug and citrulline concentrations and to determine the composition of the gut microbiota. 21 patients with a median age of 58 (interquartile range 54–64) years were included with 252 citrulline, 155 ciprofloxacin, 139 fluconazole, and 76 acyclovir concentrations and 48 fecal swabs obtained. Severe gastrointestinal mucositis was observed in all patients. Due to limited data correlation analysis was not done for valacyclovir and fluconazole, however we did observe a weak correlation between ciprofloxacin and citrulline concentrations. This could suggest that underexposure of ciprofloxacin can occur during severe mucositis. A follow-up study using frequent sampling rather than the use of left-over would be required to investigate the relationship between gastrointestinal mucositis, drug exposure, and gut microbiome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In autologous hematopoietic stem cell transplantation (HSCT) recipients, gastrointestinal mucositis is a severe complication of certain cytotoxic chemotherapeutic agents. This condition is characterized by severe ulcerations and diffuse inflammation throughout the gastrointestinal tract [1]. The agents that are most often associated with mucositis are alkylating agents (e.g., melphalan and cyclophosphamide), anthracyclines (e.g., doxorubicin), antimetabolites (e.g., 5-fluorouracil), antitumor agents (e.g., bleomycin), taxanes, and vinca alkaloids [2]. Clinically, this complication manifests as oral mucositis with ulcers and pain, diarrhea, and abdominal pain.
Due to the gastrointestinal mucositis and level of immunosuppression, HSCT recipients are highly susceptible to blood stream infections [1], which arise from translocation of pathogens from the gut and exogenous contaminants (e.g., catheters) [3]. Prophylaxis is therefore routinely used to prevent infections [4]. As gastrointestinal mucositis affects the integrity of the mucosa, a decrease of the absorptive capacity and increase of permeability occur [5], coupled with changes in the microbial ecosystem of the gastrointestinal tract [6, 7]. The selection of oral or nous drug administration in people with gastrointestinal mucositis may have clinical consequences.
It is poorly understood how gastrointestinal mucositis may affect anti-infective drug absorption because of the variable and semiquantitative assessment of mucositis in daily practice as this has not been studied [8]. We do, however, hypothesize that intestinal barrier disruption during gastrointestinal mucositis could lead to an impaired intestinal barrier function, resulting in the alteration of the efficacy of anti-infectives commonly administrated in cancer patients. Plasma citrulline—a biomarker of enterocyte mass—can be used to objectively monitor and quantify gastrointestinal mucositis severity [9, 10]. Citrulline is a nonprotein amino acid produced in the enterocytes by glutamine, which has been increasingly shown to correlate with the intestinal villus length and with radiation-induced and chemotherapy-induced (e.g., methotrexate and melphalan) mucositis in mouse and rat models [11,12,13]. In HSCT recipients, low citrulline concentrations have been related to severe mucosal barrier injury and showed a strong correlation with the daily gut score [14,15,16].
Moreover, given the lack of consensus and insight into how gastrointestinal mucositis and secondary microbiome disruption influence the absorption and bioavailability of antimicrobial drugs [17, 18], the aim of our study was to investigate the relationship between plasma concentrations of ciprofloxacin, fluconazole, and valacyclovir, with gastrointestinal mucositis severity (defined by citrulline) and gut microbiome composition.
Materials and methods
Study design
A prospective, observational pilot study was performed at the Department of Hematology, University Medical Center Groningen (UMCG), Groningen, the Netherlands. Patients aged ≥ 18 years undergoing HSCT and receiving anti-infective prophylaxis (ciprofloxacin, fluconazole, or valacyclovir) as routine clinical care were eligible for inclusion. Patients deemed unsuitable to participate at inclusion (e.g., critical illness resulting in severely reduced life expectancy) as judged by the attending physician were not included in the study. Written informed consent was obtained from each patient. The study was evaluated by the Medical Ethics Committee of UMCG and considered to have a negligible risk due to the observational nature of the study (METc 2019/073).
The primary objective was to describe the exposure of the previously mentioned anti-infectives during different stages of mucositis (as defined by citrulline concentrations) and analyze the relationship between anti-infective drug and citrulline concentrations. The secondary outcome was to investigate the composition of the gut microbiota at the baseline level and during mucositis and to explore a potential relationship between gut microbiota and the exposure of anti-infectives.
Data and sample collection
Available left-over blood samples from routine care were collected daily from the day of transplant up to 14 days after autologous stem cell transplantation. EDTA tubes containing left-over blood were centrifuged at 2000 rcf for 5 min. The resultant plasma was used to measure ciprofloxacin, fluconazole, acyclovir, and citrulline concentrations (drug bioassays described in the supplementary material). The limit of quantification for acyclovir was 0.1 mg/L, for ciprofloxacin was 0.1 mg/L, and for fluconazole was 0.5 mg/L. The bioassays are further described in the supplementary materials. Available left-over fecal swabs (ESwabs, Copan Diagnostics Inc., Brescia, Italy) were collected at weekly intervals from the laboratory of Medical Microbiology and Infection Control of the UMCG in the same time period. The fecal swabs were used to characterize the composition of the gut microbiota.
The following data was collected from electronic patient files: demographic data (sex, age, weight, and height), presence of dialysis, presence of gastrointestinal tubes, documented vomiting or diarrhea, comedication, drug regimen for the analyzed anti-infective prophylaxis (dose, frequency, and route of administration), any plasma or serum concentrations of fluconazole, ciprofloxacin, or acyclovir obtained in routine care, C-reactive protein (CRP) values, and routine blood tests. Ciprofloxacin was administered twice daily as 500 mg orally or 400 mg intravenously, valacyclovir 500 mg twice daily orally, and fluconazole 200 mg once daily either orally or intravenously. Grouping of samples was performed according to previous studies that show severe mucositis symptomology after day 4 after HSCT [9, 13, 19]. As such, samples were divided according to the time of sampling with “mucositis phase I” corresponding to day − 10 (before HSCT) to 4, “mucositis phase II” corresponding to day 5–14.
Mucositis assessment
Plasma citrulline was measured in 30 μL of plasma using automated ion-exchange column chromatography (Waters, Milford, USA) as previously described [11, 14]. The precision and accuracy were reported as interday CV% of < 3.9% and recoveries ranged from 98.0 to 100%. Levels below 10 μmol/L indicated hypocitrullinemia and considered to represent severe gastrointestinal mucositis. The limit of quantification was 0.3 μmol/L with 10 μL sample.
Microbiome analysis; 16S rRNA gene sequencing
The fecal microbiota composition was assessed using 16S rRNA gene sequencing as previously described, with few modifications [20,21,22]. The analysis is further described in the supplementary materials.
Statistical analysis
Numerical variables were presented with medians and interquartile range (IQR), categorical variables by frequencies and percentages. Spearman’s correlation test was performed between all concentrations obtained on oral anti-infective therapy and citrulline values. The difference between citrulline and different mucositis phases and drug concentrations (obtained on oral therapy) and different mucositis phases were done with the Wilcoxon test, which was corrected for multiple testing. The criteria to include the drugs in the analysis were above limit of quantification, reached steady state and measurement on oral therapy.
A \(p\) value of < 0.05 was considered statistically significant and correction for multiple hypothesis testing used, except in the case of small test numbers, in which case the Benjamini-Hochberg (BH) correction was used. Statistical analysis and graphs were performed with R version 3.3.3 and 4.0.5.
Results
Patient characteristics
From September 2019 to January 2021 including a temporary recruitment stop due to the COVID-19 pandemic, 21 patients with a median age of 58 (IQR 54–64) years were included in the study. At inclusion, no patients were deemed unsuitable to participate. Eleven patients (52%) had multiple myeloma, and high-dose melphalan was the main (57%) conditioning regimen used. None of the patients were excluded due to the exclusion criteria. The majority of the patients (\(n=18\)) received concomitant benzylpenicillin, 8 patients received also ceftazidime, single patients received other intravenous anti-infectives (e.g., piperacillin-tazobactam, cefazolin, and caspofungin). The patient characteristics are presented in Table 1. The number of biospecimens ranged from 2 to 20 per patient from day − 10 to 14 with sampling time points differing from each patient. From the left-over samples (\(n=252\)), 252 citrulline, 110 ciprofloxacin, 80 fluconazole, and 68 acyclovir trough concentrations were obtained on oral therapy. Overall, 48 fecal swabs from 14 patients were analyzed.
Citrulline and anti-infective drug concentrations in plasma
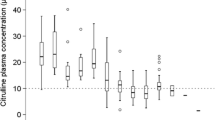
All included patients suffered from gastrointestinal mucositis as indicated by plasma citrulline concentrations. The median citrulline concentration was 20.2 (IQR 15.1–28.1) μmol/L at mucositis phase I and 9.6 (IQR 6.9–12.7) μmol/L for mucositis phase II. In all patients, a dynamic decrease in plasma citrulline levels was observed over time (Fig. 1A). Subgroup analysis showed a significant decrease in plasma citrulline concentration from mucositis phase I to mucositis phase II (Fig. 1B).
For ciprofloxacin, the median Cmin was 0.3 (range 0.1–1.6) mg/L. 66% (45 samples) of acyclovir trough concentrations were below the limit of quantification, and thus, the median was 0.1 (range 0.1–0.3) mg/L. Three patients (23%) on oral fluconazole therapy reached steady state. The overall median was 5.5 (range 0.3–14.8) mg/L.
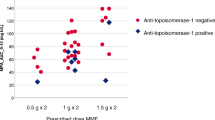
The correlation analysis was done only for ciprofloxacin as fluconazole concentrations did not reach steady state and acyclovir was measured largely under the limit of quantification. There was a significant correlation observed between ciprofloxacin and citrulline concentrations (\(R = 0.38\), p = 3.9*10−5, Fig. 2A), and a significant difference of ciprofloxacin concentrations was observed between phase I and phase II (Wilcoxon’s \(p = 0.0015\), Fig. 2B).
The influence of the gut microbiota on drug exposure
Analysis of fecal swabs was not possible for all patients, due to insufficient fecal material. Therefore, only 14 patients were included in the analysis of the gut microbiota (median of 3 samples per patient). In this cohort, contrarily to conditioning regimens with BEAM and melphalan that included a considerable number of patients (\(n=4\) and \(n=9\), respectively), conditioning regimen with cyclophosphamide and busulfan only included 1 patient. This patient was excluded from the analysis as there was not enough material to analyze trends. For this analysis, only the ciprofloxacin levels were included as these were available for the time points of the fecal swabs (Table S1).
Microbial diversity, as represented by Shannon index, throughout showed no significant differences over time (Fig. 3A). Similarly, the ordination plot based on beta diversity (Bray–Curtis distance on the taxonomic level of ASV) showed no separation of samples by mucositis phase (\(p=0.47\); ADONIS) (Fig. 3B). The relationship between ciprofloxacin exposure and individual genera/family is presented in Fig. 3C.
Dynamics of microbial diversity over time and conditioning regimen and Pearson correlation analysis between gut microbiota and plasma citrulline and anti-infective drugs. A No differences in microbial diversity were observed throughout time, as indicated by Shannon. B Linear regression shows a strong correlation between citrulline levels and day (R.2 = 0.62, \(p<0.001\)). C Heatmap of Pearson’s correlation coefficient matrix (\(R\) values are labeled and BH-corrected \(p\) values (\(p < 0.05\)) are indicated with color background)
Discussion
This pilot study attempted to describe the exposure of anti-infectives during different stages of gastrointestinal mucositis, defined by plasma citrulline concentrations. We showed significant association between ciprofloxacin concentrations and plasma citrulline concentration. Interestingly, in the 16S rRNA analysis, there was no significant alterations in microbial diversity over time and ciprofloxacin exposure did not impact any specific individual genera/family.
The analysis showed a significant but a weak correlation between ciprofloxacin and citrulline concentrations. The potential lack of a strong correlation between the parameters could be explained by the limitations in sample frequency for each drug and clinical parameter per patient. To truly assess the absorption and exposure differences of these specific anti-infectives, multiple time points per day are needed, e.g., a pharmacokinetic curve containing 3 samples based on a limited sampling strategy. In addition, the timeframe of this study was relatively short; thus, the patients did not have full recovery of mucositis during the 14 days of data collection.
Recently, the gut microbiota has received great attention due to its influence on gastrointestinal mucositis pathobiology [23,24,25]. In fact, evidence now supports the role of host-microbe interactions in the development of mucositis, with changes in its composition coinciding with the development of mucositis symptomology [5, 26]. Additionally, the gut microbiota has been increasingly recognized for its influence on the toxicity profile of anticancer agents [26]. In fact, the “TIMER” model recently proposed by Alexander et al. eloquently describes the involvement of bacteria in Translocation, Immunomodulation, Metabolism, Enzymatic degradation, and Reduced diversity, all of which have a crucial impact on treatment efficacy and toxicity [26]. As such, we investigated the role of the gut microbiome in this cohort and explored associations between bacterial composition and drug exposure as there is emerging data showing that the gut microbiota may influence the exposure of drug. However, no significant associations were observed between individual genera/family and ciprofloxacin, which may suggest that this antibiotic did not influence the gut microbiota composition, at least within the timeframe in which the samples were collected. In addition, the absence of microbiota changes could also be explained due to the absence of wide spectrum anti-infective use in the population. This result could be explained by bacteria being able to store drugs intracellularly without chemically modifying them as recently suggested by Klünemann et al. [27]. It is however important to acknowledge that the limited number of samples per patient could also explain the lack of significant alterations in the gut microbiota composition observed and therefore our interpretation on the impact of ciprofloxacin on the gut microbiota. Moreover, within the patient group, severe blood stream infections were not documented; however, the absence of these can be assumed due to the limited use of wide spectrum anti-infectives. The anti-infectives used for blood stream infections have an impact on the microbiome; thus, more severe damage might not have been present in our study population.
In this pilot study, we observed a weak correlation between ciprofloxacin and citrulline concentrations, which could suggest that underexposure of ciprofloxacin can occur during severe mucositis. A follow-up study using frequent sampling rather than the use of left-over would be required to investigate the relationship between gastrointestinal mucositis, drug exposure, and gut microbiome.
Data availability
The custom code is available on request from the principal investigator.
References
Niscola P, Romani C, Cupelli L et al (2007) Mucositis in patients with hematologic malignancies: an overview. Haematol. https://doi.org/10.3324/haematol.10232
Naidu MUR, Ramana GV, Rani PU et al (2004) Chemotherapy-induced and/or radiation therapy-induced oral mucositis–complicating the treatment of cancer. Neoplasia 6:423–431. https://doi.org/10.1593/neo.04169
Piñana JL, Montesinos P, Martino R et al (2014) Incidence, risk factors, and outcome of bacteremia following autologous hematopoietic stem cell transplantation in 720 adult patients. Ann Hematol 93:299–307. https://doi.org/10.1007/s00277-013-1872-4
Gafter-Gvili A, Fraser A, Paul M, Leibovici L (2005) Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 142:979
Bowen J, Al-Dasooqi N, Bossi P et al (2019) The pathogenesis of mucositis: updated perspectives and emerging targets. Support Care Cancer 27:4023–4033. https://doi.org/10.1007/s00520-019-04893-z
Masetti R, Zama D, Leardini D et al (2020) The gut microbiome in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer 67:e28711
Ciernikova S, Kasperova B, Drgona L et al (2021) Targeting the gut microbiome: an emerging trend in hematopoietic stem cell transplantation. Blood Rev 48:100790. https://doi.org/10.1016/j.blre.2020.100790
Gussgard AM, Hope AJ, Jokstad A et al (2014) Assessment of cancer therapy-induced oral mucositis using a patient-reported oral mucositis experience questionnaire. PLoS ONE 9:e91733–e91733. https://doi.org/10.1371/journal.pone.0091733
van der Velden WJFM, Herbers AHE, Brüggemann RJM et al (2013) Citrulline and albumin as biomarkers for gastrointestinal mucositis in recipients of hematopoietic SCT. Bone Marrow Transplant 48:977–981. https://doi.org/10.1038/bmt.2012.278
Kuiken NSS, Rings EHHM, Blijlevens NMA, Tissing WJE (2017) Biomarkers and non-invasive tests for gastrointestinal mucositis. Support Care Cancer 25:2933–2941. https://doi.org/10.1007/s00520-017-3752-2
Fijlstra M, Rings EHHM, Verkade HJ et al (2011) Lactose maldigestion during methotrexate-induced gastrointestinal mucositis in a rat model. Am J Physiol Gastrointest Liver Physiol 300:G283–G291. https://doi.org/10.1152/ajpgi.00462.2010
El-Ghazaly MA, El-Hazek RM, Khayyal MT (2015) Protective effect of the herbal preparation, STW 5, against intestinal damage induced by gamma radiation in rats. Int J Radiat Biol 91:150–156. https://doi.org/10.3109/09553002.2014.954059
Wardill HR, de Mooij CEM, da Silva Ferreira AR et al (2021) Translational model of melphalan-induced gut toxicity reveals drug-host-microbe interactions that drive tissue injury and fever. Cancer Chemother Pharmacol. https://doi.org/10.1007/s00280-021-04273-7
van Vliet MJ, Tissing WJE, Rings EHHM et al (2009) Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr Blood Cancer 53:1188–1194. https://doi.org/10.1002/pbc.22210
Blijlevens NMA, Lutgens LCHW, Schattenberg AVMB, Donnelly JP (2004) Citrulline: a potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplant 34:193–196. https://doi.org/10.1038/sj.bmt.1704563
Derikx JPM, Blijlevens NMA, Donnelly JP et al (2009) Loss of enterocyte mass is accompanied by diminished turnover of enterocytes after myeloablative therapy in haematopoietic stem-cell transplant recipients. Ann Oncol Off J Eur Soc Med Oncol 20:337–342. https://doi.org/10.1093/annonc/mdn579
Effinger A, O’Driscoll CM, McAllister M, Fotaki N (2019) Impact of gastrointestinal disease states on oral drug absorption – implications for formulation design – a PEARRL review. J Pharm Pharmacol 71:674
Kovanda LL, Marty FM, Maertens J et al (2017) Impact of mucositis on absorption and systemic drug exposure of isavuconazole. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00101-17
Stringer AM, Al-Dasooqi N, Bowen JM et al (2013) Biomarkers of chemotherapy-induced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support Care Cancer 21:1843–1852. https://doi.org/10.1007/s00520-013-1741-7
De Goffau MC, Luopajärvi K, Knip M et al (2013) Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. https://doi.org/10.2337/db12-0526
da Silva Serreira AR, Wardill HR, Havinga R et al (2021) Prophylactic treatment with vitamins c and b2 for methotrexate-induced gastrointestinal mucositis. Biomolecules 11:34. https://doi.org/10.3390/biom11010034
Yu Z, Morrison M (2004) Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36:808. https://doi.org/10.2144/04365st04
van Vliet MJ, Harmsen HJM, de Bont ESJM, Tissing WJE (2010) The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 6:1–7. https://doi.org/10.1371/journal.ppat.1000879
da Silva Ferreira AR, Wardill HR, Tissing WJE, Harmsen HJM (2020) Pitfalls and novel experimental approaches to optimize microbial interventions for chemotherapy-induced gastrointestinal mucositis. Curr Opin Support Palliat Care. https://doi.org/10.1097/SPC.0000000000000497
Logan RM, Stringer AM, Bowen JM et al (2009) Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother Pharmacol 63:239–251. https://doi.org/10.1007/s00280-008-0732-8
Alexander JL, Wilson ID, Teare J et al (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 14:356–365. https://doi.org/10.1038/nrgastro.2017.20
Klünemann M, Andrejev S, Blasche S et al (2021) Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 597:533–538. https://doi.org/10.1038/s41586-021-03891-8
Funding
Anne-Grete Märtson and Ana Rita da Silva Ferreira were funded by Marie Skłodowska-Curie Actions (grant agreement no. 712660-PRONKJEWAIL-H2020-MSCA-COFUND-2015). H.R.W. is the recipient of an NHMRC CJ Martin Biomedical Research Fellowship. L.L. is funded by China Scholarship Council (CSC201908320432) and supported in parts by the Graduate School of Medical Sciences, University of Groningen.
Author information
Authors and Affiliations
Contributions
AGM and ARSF were responsible for designing the protocol and the analysis plan, including patients and collecting the samples from the laboratory, extracting and analyzing data, interpreting results, and writing the manuscript. AV was responsible for recruitment of patients, interpreting results, and writing the manuscript. LL was responsible for analyzing and interpreting the results, statistical analysis, and writing the manuscript. HRW was responsible for interpreting the results and writing and evaluating the manuscript. LATJ was responsible for laboratory analysis of the plasma to obtain drug concentrations, interpreting results, and writing the manuscript. TSW, HJMH, and MGGS were responsible for interpreting results and writing the manuscript. LFS, WJET, and JWCA were responsible for designing the protocol and the analysis plan, interpreting results, and writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was evaluated by the Medical Ethics Committee of UMCG and considered to have a negligible risk due to the observational nature of the study (METc 2019/073).
Consent to participate
Written informed consent was obtained from the parents.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Märtson, AG., da Silva Ferreira, A.R., Veringa, A. et al. Exposure of anti-infective drugs and the dynamic changes of the gut microbiota during gastrointestinal mucositis in autologous stem cell transplant patients: a pilot study. Ann Hematol 102, 421–427 (2023). https://doi.org/10.1007/s00277-023-05091-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05091-y