Abstract
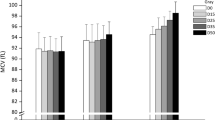
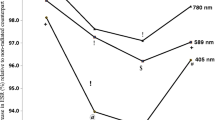
The aim of the present work was to evaluate the redox and oligomeric effects associated with the human hemoglobin of stored red blood cells that had been previously submitted to gamma radiation. Whole blood was collected from healthy donors and irradiated with 25 Gy of γ-radiation within 24 h of collection. At days 3, 5, 7, 9, 11, 14, and 28 postirradiation, fractions were removed and centrifuged, and the levels of methehemoglobin and oxyhemoglobin were measured. Hb was isolated to measure the denaturation and UV–vis spectra. The results from electrophoresis demonstrated that there was no fragmentation or cross-linking of the hemoglobin. However, ferrous center oxidation was identified as a very significant process. This mechanism is likely through an autoxidation process of the ferrous heme center, which has a maximal intensity between 5 and 7 days of storage. Interestingly, a subsequent reduction of the oxidized heme species was observed, and after 9 days of storage, the difference between the ferric species present in the control and irradiated samples was not representative. This interesting fact suggests a type of “protective action” by the blood to control the oxidative stress generated by the gamma irradiation. The UV–vis measurements demonstrated that the oxidized species was predominantly formed by hemichrome species (bis-histidine ferric heme species), which are usually associated with Heinz bodies. After 28 days of storage, evidence from the UV–vis measurements indicated that the oxidation of the irradiated sample was much higher than that observed in the control sample. These results demonstrate that despite the minimal polypeptide changes observed in the hemoglobin of stored red blood cells after gamma irradiation, the oxidation of the heme metallic center is not irrelevant and must be controlled to improve the hematological clinical procedures associated with the storage of red blood cells.





Similar content being viewed by others
References
Reiser H, Stadecker MJ (1996) Costimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N Engl J Med 335(18):1369–1377. doi:10.1056/NEJM199610313351807
Billingham RE (1966) The biology of graft-versus-host reactions. Harvey Lect 62:21–78
Mathe G, Bernard J, de Vries M, Schwarzenberg L, Larrieu MJ, Lalanne CM, Dutreix A, Amiel JL, Surmont J (1960) New trials with homologous bone marrow grafts after total irradiation in children with acute leukemia in remission. The problem of the secondary syndrome in man. Rev Hematol 15:115–161
Szweda-Lewandowska Z, Krokosz A, Gonciarz M, Zajeczkowska W, Puchała M (2003) Damage to human erythrocytes by radiation-generated HO* radicals: molecular changes in erythrocyte membranes. Free Radic Res 37(10):1137–1143
Abuja PM, Albertini R (2001) Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta 306(1–2):1–17
Moreira OC, Oliveira VH, Benedicto LB, Nogueira CM, Mignaco JA, Fontes CF, Barbosa LA (2008) Effects of gamma-irradiation on the membrane ATPases of human erythrocytes from transfusional blood concentrates. Ann Hematol 87(2):113–119. doi:10.1007/s00277-007-0378-3
Góes EG, Ottoboni MA, Palma PV, Morais FR, Pelá CA, Borges JC, Covas DT (2008) Quality control of blood irradiation with a teletherapy unit: damage to stored red blood cells after cobalt-60 gamma irradiation. Transfusion 48(2):332–340. doi:10.1111/j.1537-2995.2007.01527.x
Eder HA, Finch C, McKEE RW (1949) Congenital methemoglobinemia; a clinical and biochemical study of a case. J Clin Invest 28(2):265–272
Karon BS, Van Buskirk CM, Jaben EA, Hoyer JD, Thomas DD (2012) Temporal sequence of major biochemical events during blood bank storage of packed red blood cells. Blood Transfus 10:1–9. doi:10.2450/2012.0099-11
Jaben EA, vBC M (2011) Morphologic changes of red blood cells during blood bank storage at 1–6 degrees Celsius for 42 days. Transfusion 51
Berezina TL, Zaets SB, Morgan C, Spillert CR, Kamiyama M, Spolarics Z, Deitch EA, Machiedo GW (2002) Influence of storage on red blood cell rheological properties. J Surg Res 102(1):6–12. doi:10.1006/jsre.2001.6306
Mukherjee S, Marwaha N, Prasad R, Sharma RR, Thakral B (2010) Serial assessment of biochemical parameters of red cell preparations to evaluate safety for neonatal transfusions. Indian J Med Res 132:715–720
Yoshida T, Shevkoplyas SS (2010) Anaerobic storage of red blood cells. Blood Transfus 8(4):220–236. doi:10.2450/2010.0022-10
Kanias T, Acker JP (2010) Biopreservation of red blood cells—the struggle with hemoglobin oxidation. FEBS J 277(2):343–356. doi:10.1111/j.1742-4658.2009.07472.x
Karon BS, van Buskirk CM, Jaben EA, Hoyer JD, Thomas DD (2012) Temporal sequence of major biochemical events during blood bank storage of packed red blood cells. Blood Transfus 10(4):453–461. doi:10.2450/2012.0099-11
Walpurgis K, Kohler M, Thomas A, Wenzel F, Geyer H, Schanzer W, Thevis M (2012) Storage-induced changes of the cytosolic red blood cell proteome analyzed by 2D DIGE and high-resolution/high-accuracy MS. Proteomics 12(21):3263–3272. doi:10.1002/pmic.201200280
Cluitmans JC, Hardeman MR, Dinkla S, Brock R, Bosman GJ (2012) Red blood cell deformability during storage: towards functional proteomics and metabolomics in the blood bank. Blood Transfus 10(Suppl 2):s12–s18. doi:10.2450/2012.004S
Sekeroglu MR, Huyut Z, Him A (2012) The susceptibility of erythrocytes to oxidation during storage of blood: effects of melatonin and propofol. Clin Biochem 45(4–5):315–319. doi:10.1016/j.clinbiochem.2011.12.021
Moroff G, George VM, Siegl AM, Luban NL (1986) The influence of irradiation on stored platelets. Transfusion 26(5):453–456
Zimmermann R, Schoetz AM, Frisch A, Hauck B, Weiss D, Strobel J, Eckstein R (2011) Influence of late irradiation on the in vitro RBC storage variables of leucoreduced RBCs in SAGM additive solution. Vox Sang 100(3):279–284. doi:10.1111/j.1423-0410.2010.01410.x
Evelyn KA, Malloy HT (1938) Microdetermination of oxyhemoglobin, methemoglobin and sulfhemoglobin in a single sample of blood. J Biol Chem 126:7
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Boylston M, Beer D (2002) Methemoglobinemia: a case study. Crit Care Nurse 22(4):50–55
Coletta M, Amiconi G, Bellelli A, Bertollini A, Carsky J, Castagnola M, Condò S, Brunori M (1988) Alteration of T-state binding properties of naturally glycated hemoglobin, HbA1c. J Mol Biol 203(1):233–239
Moreira LM, Santiago PS, de Almeida EV, Tabak M (2008) Interaction of giant extracellular Glossoscolex paulistus hemoglobin (HbGp) with zwitterionic surfactant N-hexadecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (HPS): effects of oligomeric dissociation. Colloids Surf B Biointerfaces 61(2):153–163. doi:10.1016/j.colsurfb.2007.07.010
Poli AL, Moreira LM, Hidalgo AA, Imasato H (2005) Autoxidation studies of extracellular hemoglobin of Glossoscolex paulistus at pH 9: cyanide and hydroxyl effect. Biophys Chem 114(2–3):253–260. doi:10.1016/j.bpc.2004.12.041
Poli AL, Moreira LM, Tabak M, Imasato H (2006) SDS (sodium dodecyl sulfate) effect on the autoxidation of the Glossoscolex paulistus giant extracellular hemoglobin: kinetic studies at pH 7.0 and 9.0. Colloids Surf B Biointerfaces 52(1):96–104. doi:10.1016/j.colsurfb.2006.07.010
Zhu H, Ownby DW, Riggs CK, Nolasco NJ, Stoops JK, Riggs AF (1996) Assembly of the gigantic hemoglobin of the earthworm Lumbricus terrestris. Roles of subunit equilibria, non-globin linker chains, and valence of the heme iron. J Biol Chem 271(47):30007–30021
Shibata T, Nagao S, Fukaya M, Tai H, Nagatomo S, Morihashi K, Matsuo T, Hirota S, Suzuki A, Imai K, Yamamoto Y (2010) Effect of heme modification on oxygen affinity of myoglobin and equilibrium of the acid-alkaline transition in metmyoglobin. J Am Chem Soc 132(17):6091–6098. doi:10.1021/ja909891q
Moreira LM, Poli AL, Costa-Filho AJ, Imasato H (2008) Ferric species equilibrium of the giant extracellular hemoglobin of Glossoscolex paulistus in alkaline medium: HALS hemichrome as a precursor of pentacoordinate species. Int J Biol Macromol 42(2):103–110. doi:10.1016/j.ijbiomac.2007.10.001
Moreira LM, Poli AL, Costa AJ, Imasato H (2006) Pentacoordinate and hexacoordinate ferric hemes in acid medium: EPR, UV–vis and CD studies of the giant extracellular hemoglobin of Glossoscolex paulistus. Biophys Chem 124(1):62–72. doi:10.1016/j.bpc.2006.05.030
Shikama K, Matsuoka A (2003) Human haemoglobin: a new paradigm for oxygen binding involving two types of alphabeta contacts. Eur J Biochem 270(20):4041–4051
Brantley RE, Smerdon SJ, Wilkinson AJ, Singleton EW, Olson JS (1993) The mechanism of autooxidation of myoglobin. J Biol Chem 268(10):6995–7010
Griffon N, Baudin V, Dieryck W, Dumoulin A, Pagnier J, Poyart C, Marden MC (1998) Tetramer-dimer equilibrium of oxyhemoglobin mutants determined from auto-oxidation rates. Protein Sci 7(3):673–680
Marden MC, Griffon N, Poyart C (1995) Oxygen delivery and autoxidation of hemoglobin. Transfus Clin Biol 2(6):473–480. doi:10.1016/S1246-7820(05)80074-6
Pawlak AL (1977) Effect of ligands of ferric hemes on interaction between ferric and ferrous chains in partially oxidized hemoglobin-A. Acta Biol Med Ger 36(5–6):621–624
Tada T, Watanabe Y, Matsuoka A, Ikeda-Saito M, Imai K, Yukio N, Shikama K (1998) African elephant myoglobin with an unusual autoxidation behavior: comparison with the H64Q mutant of sperm whale myoglobin. Bba-Protein Struct M 1387(1–2):165–176. doi:10.1016/S0167-4838(98)00118-6
Tsuruga M, Matsuoka A, Hachimori A, Sugawara Y, Shikama K (1998) The molecular mechanism of autoxidation for human oxyhemoglobin. Tilting of the distal histidine causes nonequivalent oxidation in the beta chain. J Biol Chem 273(15):8607–8615
Acknowledgments
The authors are grateful to FAPEMIG (Fundação de Amparo à Pesquisa do estado de Minas Gerais) and to hemotherapy service of Fundação HemoMinas - Hemonúcleo Divinópolis for RBC supply.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira, G.C., Maia, G.A.S., Cortes, V.F. et al. The effect of γ-radiation on the hemoglobin of stored red blood cells: the involvement of oxidative stress in hemoglobin conformation. Ann Hematol 92, 899–906 (2013). https://doi.org/10.1007/s00277-013-1719-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1719-z