Abstract
Purpose
To evaluate the clinical utility of bland arterial embolization using microspheres in patients with hypervascular liver metastases refractory to standard treatments.
Materials and Methods
Primary endpoints of this prospective single-arm non-comparative study were objective response and disease control rates (ORR and DCR), based on the modified Response Evaluation Criteria in Solid Tumors at 4 weeks after embolization. Secondary endpoints were ORR according to primary tumor, overall survival, progression-free survival (PFS), and safety.
Results
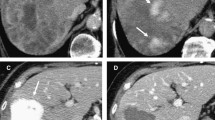
Twenty-five patients with a median age of 66 years (range, 40–95 years) were enrolled in this study. The median maximum diameter of liver metastasis was 3.7 cm (range, 2.0–15.2 cm). Primary lesions were colorectal cancer in 12 patients (48%, 12/25), other cancer in 7 (28%, 7/25), neuroendocrine tumor in 4 (16%, 4/25), and sarcoma in 2 (8%, 2/25). ORR and DCR were 52% (13/25) and 72% (18/25) in all patients, 42% (5/12) and 75% (9/12) in colorectal cancer patients, and 62% (8/13) and 69% (9/13) in other malignant tumor patients (p = 0.43, p > 0.99). Median survival time was 19 months in all patients, 19 months in colorectal cancer patients, and 8 months (p = 0.16) in other malignant tumor patients. Median PFS time was 4 months in all patients, 4 months in colorectal cancer patients, and 6 months (p = 0.0085) in other malignant tumor patients. There were no grade-3 or -4 adverse events.
Conclusion
Microsphere embolization appears to be an effective and safe treatment for hypervascular liver metastases refractory to standard treatments.


Similar content being viewed by others
References
de Ridder J, de Wilt JH, Simmer F, Overbeek L, Lemmens V, Nagtegaal I. Incidence and origin of histologically confirmed liver metastases: an explorative case-study of 23,154 patients. Oncotarget. 2016;7:55368–76.
Bengtsson G, Carlsson G, Hafström L, Jönsson PE. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg. 1981;141:586–9.
Ren Y, Dai C, Zheng H, et al. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget. 2016;7:53245–53.
He X, Zhang Q, Feng Y, et al. Resection of liver metastases from breast cancer: a multicentre analysis. Clin Transl Oncol. 2020;22:512–21.
Maluccio MA, Covey AM, Schubert J, et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer. 2006;107:1617–23.
Maire F, Lombard-Bohas C, O’Toole D, et al. Hepatic arterial embolization versus chemoembolization in the treatment of liver metastases from well-differentiated midgut endocrine tumors: a prospective randomized study. Neuroendocrinology. 2012;96:294–300.
Pitt SC, Knuth J, Keily JM, et al. Hepatic neuroendocrine metastases: chemo- or bland embolization? J Gastrointest Surg. 2008;12:1951–60.
Fiore F, Del Prete M, Franco R, et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine. 2014;47:177–82.
Tanaka T, Nishiofuku H, Maeda S, et al. Repeated bland-TAE using small microspheres injected via an implantable port-catheter system for liver metastases: an initial experience. Cardiovasc Intervent Radiol. 2014;37:493–7.
Strosberg JR, Choi J, Cantor AB, Kvols LK. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006;13:72–8.
Ruutiainen AT, Soulen MC, Tuite CM, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18:847–55.
Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104:1590–602.
Laurent A. Microspheres and nonspherical particles for embolization. Tech Vasc Interv Radiol. 2007;10:248–56. https://doi.org/10.1053/j.tvir.2008.03.010.
Osuga K, Maeda N, Higashihara H, et al. Current status of embolic agents for liver tumor embolization. Int J Clin Oncol. 2012;17:306–15.
Osuga K, Nakajima Y, Sone M, Arai Y, Nambu Y, Hori S. Transarterial embolization of hypervascular tumors using trisacryl gelatin microspheres (Embosphere): a prospective multicenter clinical trial in Japan. Jpn J Radiol. 2016;34:366–75.
Akinduro OO, Mbabuike N, ReFaey K, et al. Microsphere embolization of hypervascular posterior fossa tumors. World Neurosurg. 2018;109:182–7.
Doucet J, Kiri L, O’Connell K, et al. Advances in degradable embolic microspheres: a state of the art review. J Funct Biomater. 2018;9:14.
Osuga K, Hori S, Hiraishi K, et al. Bland embolization of hepatocellular carcinoma using superabsorbent polymer microspheres. Cardiovasc Intervent Radiol. 2008;31:1108–16.
Seki A, Hori S, Shimono C. Management of vascular lake phenomenon on angiography during chemoembolization with superabsorbent polymer microspheres. Jpn J Radiol. 2015;33:741–8.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Washington, DC: US Department of Health and Human Services, 2010.
Samaras P, Breitenstein S, Haile SR, et al. Selective intra-arterial chemotherapy with floxuridine as second- or third-line approach in patients with unresectable colorectal liver metastases. Ann Surg Oncol. 2011;18:1924–31.
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Rothenberg ML, Cox JV, Butts C, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol. 2008;19:1720–6.
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12.
Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–29.
Takaki H, Litchman T, Covey A, et al. Hepatic artery embolization for liver metastasis of gastrointestinal stromal tumor following imatinib and sunitinib therapy. J Gastrointest Cancer. 2014;45:494–9.
Brown DB, Fundakowski CE, Lisker-Melman M, et al. Comparison of MELD and Child–Pugh scores to predict survival after chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2004; 15:1209–18.
Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–9.
Mondazzi L, Bottelli R, Brambilla G, et al. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19:1115–23.
Ikeda M, Okada S, Yamamoto S, et al. Prognostic factors in patients with hepatocellular carcinoma treated by transcatheter arterial embolization. Jpn J Clin Oncol. 2002;32:455–60.
White JA, Redden DT, Bryant MK, et al. Predictors of repeat transarterial chemoembolization in the treatment of hepatocellular carcinoma. HPB (Oxford). 2014;16:1095–101.
Yamakado K, Miyayama S, Hirota S, et al. Hepatic arterial embolization for unresectable hepatocellular carcinomas: do technical factors affect prognosis? Jpn J Radiol. 2012;30:560–6.
Pelage JP, Le Dref O, Beregi JP, et al. Limited uterine artery embolization with tris-acryl gelatin microspheres for uterine fibroids. J Vasc Interv Radiol. 2003;14:15–20.
Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324–31.
Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412–25.
Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol. 2012;23:96–105.
Cianni R, Urigo C, Notarianni E, et al. Selective internal radiation therapy with SIR-spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc Intervent Radiol. 2009;32:1179–86.
Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol. 2008;19:1187–95.
Nace GW, Steel JL, Amesur N, et al. Yttrium-90 radioembolization for colorectal cancer liver metastases: a single institution experience. Int J Surg Oncol. 2011;2011:571261.
Acknowledgement
We thank the members of the study committee for response evaluation (Hiroshi Kondo, MD, Takashi Yamanaka, MD, and Atsuhiro Nakatsuka, MD), and all the investigators of this trial. This work was supported by JSPS KAKENHI (Grant Number 16K10371).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We do not have any Conflict of Interest.
Ethical Approval
The study protocol, which was approved by the ethics committees of all institutions, was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. This study was registered as Clinical Trials Registry no. UMIN000019848 (www.umin.ac.jp/ctr/index.htm).
Consent for Publication
Consent for publication was obtained by all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimohira, M., Sato, Y., Yasumoto, T. et al. Arterial Embolization Using Microspheres for Hypervascular Liver Metastases Refractory to Standard Treatments: A Multicenter Prospective Clinical Trial. Cardiovasc Intervent Radiol 44, 392–400 (2021). https://doi.org/10.1007/s00270-020-02673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02673-5