Abstract
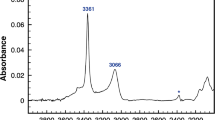
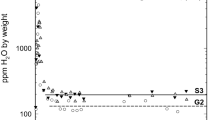
Water incorporation in forsterite samples synthesized under low to medium silica-activity conditions mostly occurs via a substitutional mechanism in which a Si vacancy is compensated by four protons. Corresponding IR absorption spectra display a cluster of narrow and weakly anharmonic OH-stretching bands at wavenumbers above 3,500 cm−1. However, this diagnostic spectrum is often superimposed to one broader absorption band, rarely two, displaying pronounced temperature-dependent properties and tentatively assigned to H atoms in interstitial position (Ingrin et al. in Phys Chem Miner 40:499–510, 2013). Here, we investigate the structural and vibrational properties of selected interstitial H-bearing defects in forsterite using a first-principles modeling approach. We show that the broad bands discussed by Ingrin et al. (Phys Chem Miner 40:499–510, 2013) are most likely related to interstitial OH groups in the vacant octahedral sites alternating with the M2 sites along the c axis of the forsterite structure. The corresponding OH defects lead to the formation of fivefold coordinated Si species. Their peculiar thermal properties stem from the vibrational phase relaxation due to the anharmonic coupling of the high-energy local OH-stretching mode with a low-energy vibrational mode. This “exchange mode” corresponds to the hindered longitudinal translation of the OH group. These results suggest that at high pressure, hydrogen incorporation in forsterite is dominated by coexisting interstitial OH groups and (4H)Si defects.





Similar content being viewed by others
References
Aubaud C, Withers AC, Hirschmann MM, Guan Y, Leshin LA, Mackwell SJ, Bell DR (2007) Intercalibration of FTIR and SIMS for hydrogen measurements in glasses and nominally anhydrous minerals. Am Mineral 92:811–828
Aubaud C, Bureau H, Raepsaet C, Khodja H, Withers AC, Hirschmann MM, Bell DR (2009) Calibration of the infrared molar absorption coefficients for H in olivine, clinopyroxene and rhyolitic glass by elastic recoil detection analysis. Chem Geol 260:286–294
Bai Q, Kohlstedt DL (1993) Effects of chemical environment on the solubility and incorporation mechanism for hydrogen in olivine. Phys Chem Minerals 19:460–471
Balan E, Lazzeri M, Delattre S, Meheut M, Refson K, Winkler B (2007) Anharmonicity of inner-OH stretching modes in hydrous phyllosilicates: assessment from first-principles frozen-phonon calculations. Phys Chem Miner 34:621–625
Balan E, Refson K, Blanchard M, Delattre S, Lazzeri M, Ingrin J, Mauri F, Wright K, Winkler B (2008) Theoretical infrared absorption coefficient of OH groups in minerals. Am Mineral 93:950–953
Balan E, Ingrin J, Delattre S, Kovacs I, Blanchard M (2011) Theoretical infrared spectrum of OH-defects in forsterite. Eur J Mineral 23:285–292
Balan E, Blanchard M, Yi H, Ingrin J (2013) Theoretical study of OH-defects in pure enstatite. Phys Chem Miner 40:41–50
Bali E, Bolfan-Casanova N, Koga KT (2008) Pressure and temperature dependence of H solubility in forsterite: an implication to water activity in the Earth interior. Earth Planet Sci Lett 268:354–363
Baroni S, de Gironcoli S, Dal Corso A, Giannozzi P (2001) Phonons and related crystal properties from density-functional perturbation theory. Rev Mod Phys 73:515–561
Bell DR, Rossman GR (1992) Water in the earth’s mantle; the role of nominally anhydrous minerals. Science 255:1391–1397
Bell DR, Rossman GR, Maldener J, Endisch D, Rauch F (2003) Hydroxide in olivine: a quantitative determination of the absolute amount and calibration of the IR spectrum. J Geophys Res 108:2105–2113
Beran A, Putnis A (1983) A model of the OH positions in olivine derived from infrared-spectroscopic investigations. Phys Chem Minerals 9:57–60
Berry AJ, Hermann J, HStC O’Neill, Foran GJ (2005) Fingerprinting the water site in mantle olivine. Geology 33:869–872
Berry AJ, O`Neill HStC, Hermann J, Scott DR (2007) The infrared signature of water associated with trivalent cations in olivine. Earth Planet Sci Lett 261:134–142
Blanchard M, Balan E, Wrigth K (2009) Incorporation of water in iron-free ringwoodite: a first-principles study. Am Miner 94:83–89
Blanchard M, Roberge M, Balan E, Fiquet G, Bureau H (2013) Infrared signatures of OH-defects in wadsleyite: a first-principles study. Am Miner. doi:10.2138/am.2013.4468
Bouhifd MA, Andrault D, Fiquet G, Richet P (1996) Thermal expansion of forsterite up to the melting point. Geophys Res Lett 23:1143–1146
Braithwaite JS, Wright K, Catlow CRA (2003) A theoretical study of the energetics and IR frequencies of hydroxyl defects in forsterite. J Geophys Res 108:2284
Brodholt JP, Refson K (2000) An ab initio study of hydrogen in forsterite and a possible mechanism for hydrolytic weakening. J Geophys Res 105:18977–18982
Budde M, Parks Cheney C, Lüpke G, Tolk NH, Feldman LCL (2001) Vibrational dynamics of bond-center hydrogen in crystalline silicon. Phys Rev B 63:195203
Bureau H, Trocellier P, Shaw C, Khodja H, Bolfan-Casanova N, Demouchy S (2003) Determination of the concentration of water dissolved in glasses and minerals using nuclear microprobe. Nucl Inst Methods Phys Res B 210:449–454
Bureau H, Raepsaet C, Khodja H, Carraro A, Aubaud C (2009) Determination of hydrogen content in geological samples using elastic recoil detection analysis (ERDA). Geochim Cosmochim Acta 73:3311–3322
Costa F, Chakraborty S (2008) The effect of water on Si and O diffusion rates in olivine and implications for transport properties and processes in the upper mantle. Phys Earth Planet Int 166:11–29
Fujino K, Sasaki S, Takeuchi Y, Sadanaga R (1981) X-ray determination of electron distributions in forsterite, fayalite and tephroite. Acta Cryst B 37:513–518
Gérard O, Jaoul O (1989) Oxygen diffusion in San Carlos olivine. J Geophys Res 94:4119–4128
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I, Dal Corso A, de Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen AP, Smogunov A, Umari P, Wentzcovitch RM (2009) Quantum ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21:395502
Gose J, Schmädicke E, Markowitz M, Beran A (2009) OH point defects in olivine from Pakistan. Miner Petrol 99:105–111
Grant KJ, Brooker RA, Kohn SC, Wood BJ (2007) The effect of oxygen fugacity on hydroxyl concentrations and speciation in olivine: implications for water solubility in the upper mantle. Earth Planet Sci Lett 261:219–229
Haiber M, Ballone P, Parrinello M (1997) Structure and dynamics of protonated Mg2SiO4: an ab initio molecular dynamics study. Am Miner 82:913–922
Harris CB, Shelby RM, Cornelius PA (1978) Intermolecular energy exchange as a mechanism for vibrational dephasing in polyatomic molecules. Chem Phys Lett 57:8–14
Hermansson K (1995) O-H bonds in electric fields: electron densities and vibrational frequency shifts. Chem Phys Lett 233:376–382
Ingrin J, Liu J, Depecker C, Kohn SC, Balan E, Grant KJ (2013) Low-temperature evolution of OH bands in synthetic forsterite, implication for the nature of H-defects at high pressure. Phys Chem Miner 40:499–510
Jacobsen SD (2006) Effect of water on the equation of state of nominally anhydrous minerals. In: Keppler H, Smyth JR (eds) Water in nominally anhydrous minerals. Reviews in mineralogy and geochemistry, vol 62. The Mineralogical Society of America, Chantilly, Virginia, pp 321–342
Jaoul O, Froidevaux C, Durham WB, Michaut M (1980) Oxygen self-diffusion in forsterite: implications for the high-temperature creep mechanism. Earth Planet Sci Lett 47:391–397
Karato S (2006) Remote sensing of hydrogen in Earth’s mantle. In: Keppler H, Smyth JR (eds) Water in nominally anhydrous minerals. Reviews in mineralogy and geochemistry, vol 62. The Mineralogical Society of America, Chantilly, Virginia, pp 343–375
Koch-Müller M, Rhede D (2010) IR absorption coefficients for water in nominally anhydrous high-pressure minerals. Am Miner 95:770–775
Koch-Müller M, Matsyuk SS, Rhede D, Wirth R, Khisina N (2006) Hydroxyl in mantle olivine xenocrysts from the Udachnaya kimberlite pipe. Phys Chem Minerals 33:276–287
Kovács I, Hermann J, O`Neill HStC, Fitz Gerald JD, Sambridge M, Horváth G (2008) Quantitative absorbance spectroscopy with unpolarized light, Part II: experimental evaluation and development of a protocol for quantitative analysis of mineral IR spectra. Am Miner 93:765–778
Kovács I, O’Neill HStC, Hermann J, Hauri EH (2010) Site-specific infrared OH absorption coefficients for water substitution into olivine. Am Miner 95:292–299
Kudoh Y, Takéuchi Y (1985) The crystal structure of forsterite Mg2SiO4 under higher pressure up to 149 kb. Zeit Kristall l7l:291–302
Kudoh Y, Kuribayashi T, Kagi H, Inoue T (2006) Cation vacancy and possible hydrogen positions in hydrous forsterite, Mg1.985Si0.993H0.06O4, synthesized at 13.5 GPa and 1300°C. J Mineral Petrol Sci 101:265–269
Lemaire C, Kohn SC, Brooker RA (2004) The effect of silica activity on the incorporation mechanisms of water in synthetic forsterite: a polarized infrared spectroscopic study. Contrib Mineral Petrol 147:48–57
Li L, Wentzcovitch RM, Weidner DJ, Da Silva CRS (2007) Vibrational and thermodynamic properties of forsterite at mantle conditions. J Geophys Res 112:B05206
Libowitzky E (1999) Correlation of O–H stretching frequencies and O–H…O hydrogen bond lengths in minerals. Monatsh Chem 130:1047–1059
Libowitzky E, Beran A (1995) OH defects in forsterite. Phys Chem Miner 22:387–392
Martin KR, Blaney P, Shi G, Stavola M, Fowler WB (2006) Temperature dependence of the vibrational spectrum of a Li–OH complex in ZnO: infrared absorption experiments and theory. Phys Rev B 73:232509
Matsyuk SS, Langer K (2004) Hydroxyl in olivines from mantle xenoliths in kimberlites of the Siberian platform. Contrib Mineral Petrol 147:413–437
Matveev S, HStC O’Neill, Ballhaus C, Taylor WR, Green DH (2001) Effect of silica activity on OH-IR spectra of olivine: implications for low-αSiO2 mantle metasomatism. J Petrol 42:721–729
Matveev S, Portnyagin M, Ballhaus C, Brooker RA, Geiger CA (2005) FTIRS spectrum of phenocryst olivine as an indicator of silica saturation in magmas. J Petrol 46:605–614
Méheut M, Lazzeri M, Balan E, Mauri F (2009) Structural control over equilibrium silicon and oxygen isotopic fractionation: a first-principles density-functional theory study. Chem Geol 258:28–37
Mitev PD, Gajewski G, Hermansson K (2009) Anharmonic OH vibrations in brucite: small pressure-induced redshift in the range 0–22 GPa. Am Miner 94:1687–1697
Mosenfelder JL, Deligne NI, Asimow PD, Rossman GR (2006) Hydrogen incorporation in olivine from 2-12 GPa. Am Miner 91:285–294
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Persson BNJ, Ryberg R (1985a) Vibrational phase relaxation at surfaces: CO on Ni (111). Phys Rev Lett 54:2119–2122
Persson BNJ, Ryberg R (1985b) Brownian motion and vibrational phase relaxation at surfaces: CO on Ni (111). Phys Rev B 32:3586–3596
Ryerson JF, Duhram WB, Cherniak DJ, Lanford WA (1989) Oxygen diffusion in olivine: effect of oxygen fugacity and implications for creep. J Geophys Res 112:B05211
Schauble E (2011) First-principles estimates of equilibrium magnesium isotope fractionation in silicate, oxide, carbonate and hexaaquamagnesium (2+) crystals. Geochim Cosmochim Acta 75:844–869
Shaw DM, Tse JS (2007) Vibrational dynamics of H+-substituted forsterite: a first-principles molecular dynamics study. Am Miner 92:1593–1600
Smyth JR, Frost DJ, Nestola F, Holl CM, Bromiley G (2006) Olivine hydration in the deep upper mantle: effects of temperature and silica activity. Geophys Res Lett 33:L15301
Umemoto K, Wentzcovitch RM, Hirschmann MM, Kohlstedt DL, Withers AC (2011) A first-principles investigation of hydrous defects and IR frequencies in forsterite: the case for Si vacancies. Am Miner 96:1475–1479
Verma AK, Karki BB (2009) Ab iitio investigations of native and protonic point defects in Mg2SiO4 polymorphs under high pressure. Earth Planet Sci Lett 285:140–149
Walker AM, Wright K, Slater B (2003) A computational study of oxygen diffusion in olivine. Phys Chem Miner 30:536–545
Walker AM, Demouchy S, Wright K (2006) Computer modelling of the energies and vibrational properties of hydroxyl groups in α and β Mg2SiO4. Eur J Miner 18:529–543
Walker AM, Hermann J, Berry AJ, HStC O’Neill (2007) Three water sites in upper mantle olivine and the role of titanium in the water weakening mechanism. J Geophys Res 112:B05211
Withers A, Bureau H, Raepsaet C, Hirschmann MM (2012) Calibration of infrared spectroscopy by elastic recoil detection analysis of H in synthetic olivine. Chem Geol 334:92–98
Wright K (2006) Atomistic models of OH defects in nominally anhydrous minerals. In: Keppler H, Smyth JR (eds) Water in nominally anhydrous minerals. Reviews in mineralogy and geochemistry, vol 62. The Mineralogical Society of America, Chantilly, Virginia, pp 343–375
Acknowledgments
This work was performed using HPC resources from GENCI-IDRIS (Grant 2013-i2013041519). It was supported by the ANR grant (NT09-566853) provided to J. Ingrin.
Author information
Authors and Affiliations
Corresponding author
Appendix: Computation of anharmonic coupling parameters
Appendix: Computation of anharmonic coupling parameters
We calculate the anharmonic coupling coefficients between the OH-stretching mode and the other vibrational modes of the defective forsterite. This is done by studying the variations of the dynamical matrix coefficients when the atoms are displaced out of their equilibrium position along the normal coordinates of the OH-stretching mode.
Assuming that the exchange mode is localized in the same region as the interstitial OH group, only vibrational properties at the Brillouin zone center are considered. In this case, the coefficients of the dynamical matrix are defined by:
where m i and x iα stand for the mass of atom i and the displacement of atom i along the Cartesian coordinate α, respectively. Let us call Z liα the orthonormal eigenvectors of the dynamical matrix. The Z liα are related to the normal coordinates Q liα of the vibration mode l by \(\tilde{m}_{l}\) Z liα = (m i )1/2 Q liα . The eigenvalues ω 2 l of the dynamical matrix can be written as:
where the bra-ket products indicate the sum over the i and α indexes.
They are related to the derivatives of the total energy by:
where \( \tilde{m}_{l} \) is the effective mass of mode l. One can then obtain
Thus, from Eq. (9), the C 1122 coefficients can be obtained from the second-order derivatives of the dynamical matrix with respect to atomic displacements along the normal coordinates of the LVM. To keep the computation time under a reasonable limit, an approximate approach is used to describe the coupling between the OH-stretching LVM and the other vibrational modes of the system. In this approach, it is assumed that the mode–mode dominant coupling terms are related to atoms in a close proximity to the OH defect.
The full dynamical matrix of the OHia supercell is computed first, leading to 342 vibrational modes. The corresponding OH-stretching wavenumber is 3,585 cm−1, in excellent agreement with the result of the calculation restricted to the OH group only (3,583 cm−1). A reduced-size model of the defect (30 atoms per unit-cell) is then built using the primitive unit-cell of forsterite. This smaller model preserves the overall geometry of the defect, with the occurrence of a fivefold Si species and the OH group pointing to the neighboring O2 atom. The Oi–H bond length is almost unchanged at 0.977 Å. The Si–Oi and Mg–Oi distances increase by ~1 % to 1.72 and 1.94 Å, respectively, whereas the Oi(H)…O2 distance shortens to 2.70 Å. Consistently, a slight decrease in the OH-stretching wavenumber to 3,540 cm−1 is observed.
The second-order derivatives of the dynamical matrix coefficients with respect to atomic displacements along the LVM normal coordinates are then obtained by calculating the dynamical matrix of the small system for four finite atomic displacements λ Q OH along the OH-stretching mode normal coordinates, with λ = − 0.01, −0.005, +0.005, +0.01. Under the assumption that the second-order derivatives of the dynamical matrix coefficients can be transferred from the small to the large system, the mode–mode coupling in the large system is described by considering the displacement of these 30 atoms only. In other words, the projection in Eq. (9) is performed using the \(\frac{{\partial^{2} D_{ij\alpha \beta } }}{{\partial Q_{\text{LVM}}^{2} }}\) coefficients computed on the small system and the atomic displacements Z ex corresponding to the full dynamical matrix. The δω parameters are then obtained using Eq. (5).
Rights and permissions
About this article
Cite this article
Balan, E., Blanchard, M., Lazzeri, M. et al. Contribution of interstitial OH groups to the incorporation of water in forsterite. Phys Chem Minerals 41, 105–114 (2014). https://doi.org/10.1007/s00269-013-0628-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-013-0628-y