Abstract
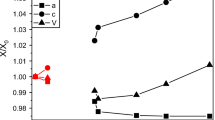
We have prepared aqueous MgSO4 solutions doped with various divalent metal cations (Ni2+, Zn2+, Mn2+, Cu2+, Fe2+, and Co2+) in proportions up to and including the pure end-members. These liquids have been solidified into fine-grained polycrystalline blocks of metal sulfate hydrate + ice by rapid quenching in liquid nitrogen. In a companion paper (Fortes et al., in Phys Chem Min 39) we reported the identification of various phases using X-ray powder diffraction, including meridianiite-structured undecahydrates, melanterite- and epsomite-structured heptahydrates, novel enneahydrates and a new octahydrate. In this work we report the changes in unit-cell parameters of these crystalline products where they exist over sufficient dopant concentrations. We find that there is a linear relationship between the rate of change in unit-cell volume as a function of dopant concentration and the ionic radius of the dopant cation; large ions such as Mn2+ produce a substantial inflation of the hydrates’ unit-cell volume, whereas smaller ions such as Ni2+ produce a modest reduction in unit-cell volume. Indeed, when the data for all hydrates are normalised (i.e., divided by the number of formula units per unit-cell, Z, and the hydration number, n), we find a quantitatively similar relationship for different values of n. Conversely, there is no relationship between the degree of unit-cell inflation or deflation and the limit to which a given cation will substitute into a certain hydrate structure; for example, Co2+ and Zn2+ affect the unit-cell volume of MgSO4·11H2O to a very similar degree, yet the solubility limits inferred in our companion paper are >60 mol. % Co2+ and <30 mol. % Zn2+.












Similar content being viewed by others
Notes
For the sake of brevity, the various species will be referred to by cation and hydration state, such that MgSO4·11H2O becomes Mg11 and CuSO4·5H2O, for example, becomes Cu5.
References
Aleksovska S, Petruševksi VM, Šoptrajanov B (1998) Calculation of structural parameters in isostructural series: the Kieserite group. Acta Crystallogr B 54(5):564–567. doi:10.1107/S0108768198000974
Anderson JL, Peterson RC, Swainson IP (2007) The atomic structure and hydrogen bonding of deuterated melanterite, FeSO4·7D2O. Can Miner 45(3):457–469. doi:10.2113/gscanmin.45.3.457
Balarew C, Karaivanova V, Aslanian S (1973) Isomorphiebeziehungen bei den Heptahydratsulfaten von einigen zweiwertigen Metallen (Mg2+, Zn2+, Ni2+, Fe2+, Co2+). Krist Tech 8:115–125. doi:10.1002/crat.19730080112
Boisen MB Jr, Gibbs GV (1990) Mathematical Crystallography: An Introduction to the Mathematical Foundations of Crystallography. Rev Miner 15:72–75
Bury CR (1924) The system zinc sulphate-water. J Chem Soc Trans 125:2538–2541. doi:10.1039/ct9242502538
Cotton FA, Falvello LR, Murillo CA, Pascual I, Schultz AJ, Tomás M (1994) Neutron and X-ray structural characterization of the hexaaquavanadium(II) compound VSO4·7H2O. Inorg Chem 33(24):5391–5395. doi:10.1021/ic00102a009
De Boisbaudran PEL (1867) Sur quelques expériences relatives à la sursaturation des dissolutions salines. Bull Soc Chim Paris (2e série) 8:3–6
Fortes AD, Wood IG, Knight KS (2008) The crystal structure and thermal expansion tensor of MgSO4·11D2O (meridianiite) determined by neutron powder diffraction. Phys Chem Miner 35(4):207–221. doi:10.1007/s00269-008-0214-x
Fortes AD, Wood IG, Fernandez-Alonso F (2011) Thermoelastic properties and high-pressure decomposition of MgSO4·11H2O (synthetic meridianiite). ISIS Experimental Report RB1110039 (OSIRIS), Rutherford Appleton Laboratory
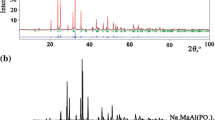
Fortes AD, Browning F, Wood IG (2012) Cation substitution in synthetic meridianiite (MgSO4·11H2O) I: X-ray powder diffraction analysis of quenched polycrystalline aggregates. Phys Chem Miner 39. doi:10.1007/s00269-012-0497-9
Giester G, Lengauer CL, Redhammer G (1994) Characterization of the FeSO4·H2O-CuSO4·H2O solid-solution series, and the nature of poitevinite. Can Miner 32:873–884
Hazen RM, Downs RT, Prewitt CT (2000) Principles of comparative crystal chemistry. Rev Miner 41:1–33
Hodenberg R, Kühn R (1967) Zur Kenntnis der Magnesiumsulfathydrate und der Effloreszenzen des Kieserits von Hartsalzen. Kali und Steinsalz 4:326–340
Iskhakova LD, Dubrovinskii LS, Charushnikova IA (1991) Crystal structure, theoretical parameters of potential atomic interaction (PPAI), and thermochemical properties of NiSO4·nH2O (n = 7, 6). Sov Phys Crystallogr 36:360–363
Jayakumari K, Mahadevan C (1992) Optical investigation on Mg x Zn1-x SO4·7H2O mixed crystals and mixed salts. J Optics 21(1):22–24
Jambor JL, Nordstrom DK, Alpers CN (2000) Metal-sulfate salts from sulfide mineral oxidation. Rev Miner 40:303–350
Jayakumari K, Mahadevan C, Chandrasekharam D (1993) X-ray investigations on Mg x Zn1−x SO4·7H2O mixed crystals. J Pure Appl Phys 5(6):331–334
Kaminski W (2004) WinTensor 1.1 (http://www.wintensor.com)
Kuz’min VG, Ivanova GV, Morozova OV, Savchenko BA (1979) Heat conductivity and thermal coefficient of linear expansion of fluorite. Meas Tech 22(8):968–969
Larson AC, Von Dreele RB (2000) General Structure Analysis System (GSAS). Los Alamos National Laboratory Report, LAUR 86–748
Lengauer CL, Giester G (1995) Rietveld refinement of the solid-solution series: (Cu, Mg)SO4·H2O. Powder Diffr 10(3):189–194
Leverett P, McKinnon AR, Williams PA (2004) New data for boothite, CuSO4·7H2O, from Burraga, New South Wales. Aust J Miner 10(1):3–6
Lœwel H (1855) Observations sur la sursaturation des dissolutions saline. III. Dissolutions sursaturées de sulfate de magnésie. Ann Chim Phys (3e série) 43:405–420
Moissan H (1882) Sur les proprietés et al. préparation du sulfate de protoxyde de chrome. Bull Soc Chim (2e série) 37:296–298
Mylius F, Funk R (1897) Ueber die Hydrate des Cadmiumsulfates. Ber Deutsch Chem Ges 30(1):824–833. doi:10.1002/cber.189703001162
Nambu M, Tanida K, Kitamura K, Kato E (1979) Mallardite from the Jôkoku Mine, Hokkaido, Japan. J Jap Assoc Miner Petrol Econ Geol 74(11):406–412
Peterson RC (2003) The relationship between Cu content and distortion in the atomic structure of melanterite from the Richmond Mine, Iron Mountains, California. Can Miner 41(4):937–949. doi:10.2113/gscanmin.41.4.937
Piccini A (1899) Über die Vanadinverbindung von der Form VX2. Z Anorg Allg Chem 19:204–207. doi:10.1002/zaac.18990190117
Piccini A, Marino L (1902) Über einige Vanadinverbindung von der Form VX2. Z Anorg Allg Chem 32:55–71. doi:10.1002/zaac.19020320105
Premkumar PS, Shajan XS (2010) X-ray and thermal studies on Zn x Mg1−x SO4·7H2O crystals. E-J Chem 7(S1):s121–s126
Ptasiewicz-Bak H, Olovsson I, McIntyre GJ (1997) Charge density in orthorhombic NiSO4·7H2O at room temperature and 25 K. Acta Crystallogr B 53(3):325–336. doi:10.1107/S0108768196014061
Redhammer GJ, Koll L, Bernroider M, Tippelt G (2007) Co2+-Cu2+ substitution in bieberite solid-solution series (Co1−xCux)SO4·7H2O, 0.00 ≤ x ≤ 0.46: Synthesis, single-crystal structure analysis, and optical spectroscopy. Am Miner 92(4):532–545. doi:10.2138/am.2007.2229
Röttger K, Endriss A, Ihringer J, Doyle S, Kuhs WF (1994) Lattice constants and thermal-expansion of H2O and D2O ice Ih between 10 and 265 K. Acta Crystallogr B 50(6):648–664. doi:10.1107/S0108768194004933
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751–767. doi:10.1107/S0567739476001551
Soma T, Kagaya H-M (1999) Thermal expansion coefficients of c-Si. In: Hull R (ed) Properties of crystalline silicon. INSPEC, The Institution of Electrical Engineers, London, pp 153–154
Theivanayagom M, Mahadevan C (2001) Lattice variations and thermal parameters of Ni x Mg1−x SO4·7H2O single crystals. Bull Mat Sci 24(5):441–444. doi:10.1007/BF02706713
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Appl Crystallogr 34(2):210–213. doi:10.1107/S0021889801002242
Wildner M, Giester G (1991) The crystal structures of Kieserite-type compounds: I. Crystal structures of Me(II)SO4·H2O (Me = Mn, Fe, Co., Ni, Zn). Neues Jb Miner Monat 7:296–306
Wood IG, Hughes N, Browning F, Fortes AD (2012) A compact, transportable, thermoelectrically-cooled cold stage for reflection geometry X-ray powder diffraction. J Appl Crystallogr (in press)
Acknowledgments
The authors thank Neil Hughes for assistance with the design and construction of the Peltier cold stage, and Kevin Knight for the use of the cryogenic pestle and mortar. This work was supported in part by Master’s student funds from the UCL Department of Earth Sciences (F. Browning), and in part by the Science and Technology Facilities Council, Fellowship number PP/E006515/1 (A. D. Fortes).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fortes, A.D., Browning, F. & Wood, I.G. Cation substitution in synthetic meridianiite (MgSO4·11H2O) II: variation in unit-cell parameters determined from X-ray powder diffraction data. Phys Chem Minerals 39, 443–454 (2012). https://doi.org/10.1007/s00269-012-0498-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-012-0498-8