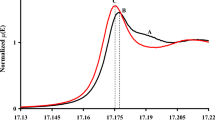
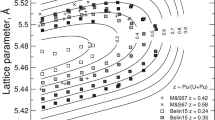
Abstract
Meridianiite, MgSO4·11H2O, is the most highly hydrated phase in the binary MgSO4–H2O system. Lower hydrates in the MgSO4–H2O system have end-member analogues containing alternative divalent metal cations (Ni2+, Zn2+, Mn2+, Cu2+, Fe2+, and Co2+) and exhibit extensive solid solution with MgSO4 and with one another, but no other undecahydrate is known. We have prepared aqueous MgSO4 solutions doped with these other cations in proportions up to and including the pure end-members. These liquids have been solidified into fine-grained polycrystalline blocks of metal sulfate hydrate + ice by rapid quenching in liquid nitrogen. The solid products have been characterised by X-ray powder diffraction, and the onset of partial melting has been quantified using a thermal probe. We have established that of the seven end-member metal sulfates studied, only MgSO4 forms an undecahydrate; ZnSO4 forms an orthorhombic heptahydrate (synthetic goslarite), MnSO4, FeSO4, and CoSO4 form monoclinic heptahydrates (syn. mallardite, melanterite, bieberite, respectively), and CuSO4 crystallises as the well-known triclinic pentahydrate (syn. chalcanthite). NiSO4 forms a new hydrate which has been indexed with a triclinic unit cell of dimensions a = 6.1275(1) Å, b = 6.8628(1) Å, c = 12.6318(2) Å, α = 92.904(2)°, β = 97.678(2)°, and γ = 96.618(2)°. The unit-cell volume of this crystal, V = 521.74(1) Å3, is consistent with it being an octahydrate, NiSO4·8H2O. Further analysis of doped specimens has shown that synthetic meridianiite is able to accommodate significant quantities of foreign cations in its structure; of the order 50 mol. % Co2+ or Mn2+, 20–30 mol. % Ni2+ or Zn2+, but less than 10 mol. % of Cu2+ or Fe2+. In three of the systems we examined, an ‘intermediate’ phase occurred that differed in hydration state both from the Mg-bearing meridianiite end-member and the pure dopant end-member hydrate. In the case of CuSO4, we observed a melanterite-structured heptahydrate at Cu/(Cu + Mg) = 0.5, which we identify as synthetic alpersite [(Mg0.5Cu0.5)SO4·7H2O)]. In the NiSO4- and ZnSO4-doped systems we characterised an entirely new hydrate which could also be identified to a lesser degree in the CuSO4- and the FeSO4-doped systems. The Ni-doped substance has been indexed with a monoclinic unit-cell of dimensions a = 6.7488(2) Å, b = 11.9613(4) Å, c = 14.6321(5) Å, and β = 95.047(3)°, systematic absences being indicative of space-group P21/c with Z = 4. The unit-cell volume, V = 1,176.59(5) Å3, is consistent with it being an enneahydrate [i.e. (Mg0.5Ni0.5)SO4·9H2O)]. Similarly, the new Zn-bearing enneahydrate has refined unit cell dimensions of a = 6.7555(3) Å, b = 11.9834(5) Å, c = 14.6666(8) Å, β = 95.020(4)°, V = 1,182.77(7) Å3, and the new Fe-bearing enneahydrate has refined unit cell dimensions of a = 6.7726(3) Å, b = 12.0077(3) Å, c = 14.6920(5) Å, β = 95.037(3)°, and V = 1,190.20(6) Å3. The observation that synthetic meridianiite can form in the presence of, and accommodate significant quantities of other ions increases the likelihood that this mineral will occur naturally on Mars—and elsewhere in the outer solar system—in metalliferous brines.










Similar content being viewed by others
References
Angel RJ, Finger LW (1988) Polymorphism of nickel sulfate hexahydrate. Acta Crystallgr C 44:1869–1873. doi:10.1107/S0108270188006717
Armunanto R, Schwenk CF, Bambang Setiaji AH, Rode BM (2003) Classical and QM/MM molecular dynamics simulations of Co2+ in water. Chem Phys 295(1):63–70. doi:10.1016/j.chemphys.2003.08.006
Arvidson RE, Poulet F, Bibring JP, Wolff M, Gendrin A, Morris RV, Freeman JJ, Langevin Y, Mangold N, Bellucci G (2005) Spectral reflectance and morphological correlations in eastern Terra Meridiani, Mars. Science 307:1591–1593. doi:10.1126/science.1109509
Aslanian S, Balarew C (1977) Über die Kristallstrukturdifferenzen der MeSO4·nH2O-Salztypen. Krist Tech 12:435–446. doi:10.1002/crat.19770120503
Aslanian S, Balarew C, Oikova T (1972) Isomorphiebeziehungen zwischen ZnSO4.7H2O, NiSO4.7H2O und CoSO4.7H2O. Krist Tech 7:525–531. doi:10.1002/crat.19720070506
Averina RA, Shevchuk VG (1967) The CuSO4–MgSO4–H2O system at 25°C. Russ J Inorg Chem 12(11):1661–1662
Balarew C, Karainova V (1975) On the isodimorphous cocrystallization in the systems FeSO4–CdSO4–H2O and FeSO4–CuSO4 H2O at 25°C. CR Acad Bulg Sci 28(11):1497–1500
Balarew C, Oikova T (1975) Thermodynamische untersuchung des Systems Zinksulfat–Kobaltsulfat–Wasser bei 25°C. Comm Bulg Acad Sci Dept Chem 8(4):640–648
Balarew C, Karainova V (1976a) Über eine Möglichkeit der Gewinnung reiner, von isodimorphen Einschlüssen freier Kristallhydrate vom Typ MSO4·nH2O. Z Anorg Allg Chem 422(2):173–178. doi:10.1002/zaac.19764220209
Balarew C, Karainova V (1976b) Isodimorphe Cokristallisation in Sulfatsystemen als Möglichkeit zur Voraussage der Existenz von Kristallhydraten des Typs MSO4·nH2O (M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+). Z Anorg Allg Chem 422(3):283–288. doi:10.1002/zaac.19764220313
Balarew C, Karainova V, Oikova T (1970) Untersuchung der Systeme Zinksulfat-Kobaltsulfat-Wasser und Zinksulfat-Nickelsulfat-Wasser bei 25,0°C. Comm Bulg Acad Sci Dept Chem 3(4):637–643
Balarew C, Dobreva P, Oikova T (1973a) Изcлeдвaнe нa cиcтeмитe MgSO4–ZnSO4–H2O, MgSO4–CoSO4–H2O, MgSO4–FeSO4–H2O пpи 25,0 °C. Jahrbuch der Hochschule für Chemische Technologie – Burgas 10:503–510
Balarew C, Karaivanova V, Aslanian S (1973b) Isomorphiebeziehungen bei den Heptahydratsulfaten von einigen zweiwertigen Metallen (Mg2+, Zn2+, Ni2+, Fe2+, Co2+). Krist Tech 8:115–125. doi:10.1002/crat.19730080112
Balarew C, Karaivanova V, Stefanov I (1984) Effect of isomorphic guest component on the host crystals structures and habits. In: Jancic JJ, de Jong EJ (eds) Industrial crystallization’84. Elsevier Science, Amsterdam, pp 335–338
Balasubramanian G, Sohail Murad S, Kappiyoor R, Puri IK (2011) Structure of aqueous MgSO4 solution: dilute to concentrated. Chem Phys Lett 508(1–3):38–42. doi:10.1016/j.cplett.2011.04.010
Baur WH (1964) On the crystal chemistry of salt hydrates. II. A neutron diffraction study of MgSO4·4H2O. Acta Crystallogr 17(7):863–869. doi:10.1107/S0365110X64002304
Baur WH, Rolin JL (1972) Salt hydrates. IX. The comparison of the crystal structure of magnesium sulfate pentahydrate with copper sulfate pentahydrate and magnesium chromate pentahydrate. Acta Crystallogr B 28(5):1448–1455. doi:10.1107/S0567740872004443
Benrath A, Blankenstein A (1933) Über Mischkristalle in der Vitriolreihe. I. Z Anorg Allg Chem 216(1):41–48. doi:10.1002/zaac.19332160108
Benrath A, Blankenstein A (1934) Über Mischkristalle in der Vitriolreihe. II. Z Anorg Allg Chem 217(2):170–174. doi:10.1002/zaac.19342170207
Benrath A, Neumann E (1939) Über Mischkristalle in der Vitriolreihe. V. Z Anorg Allg Chem 242(1):70–78. doi:10.1002/zaac.19392420108
Benrath A, Schröder W (1927) Über das Octahydrat des Magnesiumsulfats. Z Anorg Allg Chem 161(1):155–158. doi:10.1002/zaac.19271610112
Benrath A, Triemann W (1934) Über Mischkristalle in der Vitriolreihe. III. Z Anorg Allg Chem 217(4):347–352. doi:10.1002/zaac.19342170405
Bish DL, Blake DF, Sarrazin P, Treiman A, Hoehler T, Hausrath EM, Migtkandal I, Steele A (2007) Field XRD/XRF mineral analysis by the MSL CheMin instrument. In: Proceedings of 38th Lunar Planet Sci Conf: abstract #1163
Bishop JL, Parente M, Weitz CM, Noe Dobrea EZ, Calvin WM, Milliken RE, Roach LA, Murchie SL, McKeown NK, Mustard JF, and the CRISM Team (2008) Characterization of light-toned sulfate and hydrated silica layers at Juventae Chasma using CRISM, OMEGA, HiRISE, and context images. In: Proceedings of 39th Lunar Planet Sci Conf: abstract #2334
Blake DF, Vaniman D, Anderson R, Bish D, Chipera S, Chemtob S, Crisp J, Des-Marais DJ, Downs R, Farmer J, Gailhanou M, Ming D, Morris D, Stolper E, Sarrazin P, Treiman A, Yen A (2009) The Chemin mineralogical instrument on the Mars Science Laboratory mission. In: Proceedings of 40th Lunar Planet Sci Conf: abstract #1484
Boultif A, Louër D (2004) Powder pattern indexing with the dichotomy method. J Appl Crystallogr 37(5):724–731. doi:10.1107/S0021889804014876
Bury CR (1924) The system zinc sulphate–water. J Chem Soc Trans 125:2538–2541. doi:10.1039/ct9242502538
Cauët E, Bogatko S, Weare JH, Fulton JL, Schenter GK, Bylaska EJ (2010) Structure and dynamics of the hydration shells of the Zn2+ ion from ab initio molecular dynamics and combined ab initio and classical molecular dynamics simulations. J Chem Phys 132(19):194502. doi:10.1063/1.3421542
Chrétien A, Rohmer R (1934) Sur les hydrates du sulfate de nickel. CR Hebd Acad Sci 198:92–94
Crockford HD, Brawley DJ (1932) The system: CuSO4–CoSO4–H2O. J Phys Chem 36(5):1594–1596. doi:10.1021/j150335a021
D’Ans J (1933) Die Lösungsgleichgewichte der System der Salz ozeanischer Salzablagerungen. Berlin, pp 118–123
De Boisbaudran PEL (1867a) Sur quelques expériences relatives à la sursaturation des dissolutions salines. Bull Soc Chim Paris (2e série) 8:3–6
De Boisbaudran PEL (1867b) Sursaturation des solutions salines. Deuxième note. Bull Soc Chim Paris (2e série) 8:65–71
De Boisbaudran PEL (1868a) Sursaturation des solutions salines. Troisième note. Bull Soc Chim Paris (2e série) 9:191–198
De Boisbaudran PEL (1868b) Note sur la sursaturation des solutions salines. CR Hebd Acad Sci 66:497–499
De Coppet LC (1897) Ueber einige ältere Bestimmungen des Gefrierpunktes gesättigter Salzlösungen. Z Phys Chem 22:239–240
De Marignac J-ChG (1857) Recherches sur les formes cristallines et la composition chimiques de divers sels. Ann Mines (5e série) 12:1–74
De Wolff PM (1968) A simplified criterion for the reliability of a powder pattern indexing. J Appl Crystallogr 1(2):108–113. doi:10.1107/S002188986800508X
Dufet H (1878) Sur la variation des indices de réfraction dans des mélanges de sels isomorphes. CR Hebd Acad Sci 86:881–884
Dufet H (1888) Notices cristallographiques. Bull Soc Franç Min 11:215–220
Egorov AV, Komolkin AV, Lyubartsev AP, Laaksonen A (2006) First and second hydration shell of Ni2+ studied by molecular dynamics simulations. Theor Chem Acc 115(2–3):170–176. doi:10.1007/s00214-005-0050-8
Fialips CI, Carey JW, Vaniman DT, Feldman WC, Bish DL, Mellon MT (2004) Sub-surface deposits of hydrous silicates or hydrous magnesium sulfates as hydrogen reservoirs near the Martian equator: plausible or not? In: Proceedings of 35th Lunar Planet Sci Conf: abstract #2054
Fortes AD, Knight KS (2010) Thermal expansion and phase transition in magnesium sulfate trihydrate. ISIS Experimental Report RB 1010078, Rutherford Appleton Laboratory
Fortes AD, Wood IG (2012) X-ray powder diffraction analysis of a new magnesium chromate hydrate, MgCrO4·11H2O. Powder Diffr 27(1). doi:10.1017/S088571561200005X, in press
Fortes AD, Wood IG, Knight KS, Alfredsson M, Vočadlo L (2006) The thermoelastic properties of epsomite (MgSO4·7D2O) from powder neutron diffraction and ab initio simulation. Eur J Mineral 18(4):449–462. doi:10.1127/0935-1221/2006/0018-0449
Fortes AD, Wood IG, Knight KS (2008) The crystal structure and thermal expansion tensor of MgSO4·11D2O (meridianiite) determined by neutron powder diffraction. Phys Chem Miner 35(4):207–221. doi:10.1007/s00269-008-0214-x
Fortes AD, Browning F, Wood IG (2012) Cation substitution in synthetic meridianiite (MgSO4·11H2O) II: variation in unit-cell parameters determined from X-ray powder diffraction data. Paper submitted to Phys Chem Miner 39. doi:10.1007/s00269-012-0498-8
Fraenckel F (1907) Über die Existenzgebiete der Ferrosulfat-Hydrate. Z Anorg Chem 55(1):223–232. doi:10.1002/zaac.19070550119
Fritzsche CJ (1837) Ueber eine neue Verbindung der schwefelsauren Talkerde mit Wasser. Ann Phys Chem 118(12):577–580. doi:10.1002/andp.18371181211
Genceli FE, Horikawa S, Iizuka Y, Sakurai T, Hondoh T, Kawamura T, Witkamp G (2009) Meridianiite detected in ice. J Glaciol 55:117–122. doi:10.3189/002214309788608921
Gendrin A, Mangold N, Bibring J-P, Langevin Y, Gondet B, Poulet F, Bonello G, Quantin C, Mustard J, Arvidson R, LeMouélic S (2005) Sulfates in Martian layered terrains: the OMEGA/Mars express view. Science 307:1587–1591. doi:10.1126/science.1109087
Giester G, Lengauer CL, Redhammer G (1994) Characterization of the FeSO4·H2O–CuSO4·H2O solid-solution series, and the nature of poitevinite. Can Mineral 32:873–884
Gorshtein GJ (1951) Иccлeдoвaния pacпpeдeния пpимeceй пpи кpиcтaллизaции нeopгaничecкиx coлeй. TrudyVsesoyuznogo Nauchno-Issledovatelskogo Instituta Khimicheskikh Reaktikov 20:1–43
Gorshtein GI, Silanteva NI (1953) Pacпpeдeлeниe изoмopфныx и изoдимopфныx кoмпoнeнтoв мeждy твepдoй и жидкoй фaзaми пpи кpиcтaллизaции из вoдныx pacтвopoв. Zh Obsch Khim 23(8):1290–1302
Groth PH (1898) Tabellarische Übersicht der Mineralien nach Ihren Krystallographischen-Chemischen Beziehungen, 4th edn. Braunschweig
Groth PH (1908) Chemische Kristallographie, teil 2: die Anorganischen Oxo- und Sulfosalze. Leipzig
Guthrie F (1876) On salt solutions and attached water. Phil Mag (5th series) 2(10):211–225
Hawthorne FC, Groat LA, Raudsepp M, Ercit TS (1987) Kieserite, Mg(SO4)(H2O), a Titanite-group mineral. Neues Jb Miner Abh 157:121–132
Herkenhoff KE, Squyres SW, Arvidson R, Bass DS, Bell JF, Bertelsen P, Ehlmann BL, Farrand W, Gaddis L, Greeley R, Grotzinger J, Hayes AG, Hviid SF, Johnson JR, Jolliff B, Kinch KM, Knoll AH, Madsen MB, Maki JN, McLennan SM, McSween HY, Ming DW, Rice JW Jr, Richter L, Sims M, Smith PH, Soderblom LA, Spanovich N, Sullivan R, Thompson S, Wdowiak T, Weitz C, Whelley P (2004) Evidence from Opportunity’s Microscopic Imager for water on Meridiani Planum. Science 306:1727–1730. doi:10.1126/science.1105286
Hey MH (1931) On pink epsomites and fauserite. Mineral Mag 22:510–518
Hodenberg R, Kühn R (1967) Zur Kenntnis der Magnesiumsulfathydrate und der Effloreszenzen des Kieserits von Hartsalzen. Kali und Steinsalz 4:326–340
Hogenboom DL, Kargel JS, Ganasan JP, Lee L (1995) Magnesium sulfate–water to 400 MPa using a novel piezometer: densities, phase equilibria and planetological implications. Icarus 115(2):258–277. doi:10.1006/icar.1995.1096
Horgan BH, Bell JF III, Noe Dobrea EZ, Cloutis EA, Bailey DT, Craig MA, Roach LH, Mustard JF (2009) Distribution of hydrated minerals in the north polar region of Mars. J Geophys Res 114:E01005. doi:10.1029/2008JE003187
Hutton CO (1947) Nickelian epsomite from north Auckland, New Zealand. Am Min 32(9–10):553–560
Inada Y, Mohammed AM, Loeffler HH, Rode BM (2002) Hydration structure and water exchange reaction of nickel(II) ion: classical and QM/MM simulations. J Phys Chem A 106(29):6783–6791. doi:10.1021/jp0155314
International Critical Tables of Numerical Data, Physics, Chemistry and Technology, vol IV. National Research Council of the USA, McGraw-Hill (1928)
Jambor JL, Nordstrom DK, Alpers CN (2000) Metal-sulfate salts from sulfide mineral oxidation. Rev Mineral 40:303–350. doi:10.2138/rmg.2000.40.6
Jangg G, Gregori H (1967) Kristallisationsgleichgewichte in den Systemen CuSO4–NiSO4–H2O, CuSO4–ZnSO4–H2O, NiSO4–ZnSO4–H2O. Z Anorg Allg Chem 351(1–2):81–99. doi:10.1002/zaac.19673510112
Kargel JS (1991) Brine volcanism and the interior structures of icy satellites. Icarus 94(368):390. doi:10.1016/0019-1035(91)90235-L
Kasatkin IA, Treivus EB, Ivanova TI (2000) Solubility isotherm in the system MgSO4–NiSO4–H2O at 25°C. Russ J Appl Chem 73(11):1880–1882
Kopp H (1863) Ueber den Pleomorphismus der schwefelsauren Magnesia MgO, SO3 + 7HO. Ann Chem Pharm 325:369–371
Kuz’min VG, Ivanova GV, Morozova OV, Savchenko BA (1979) Heat conductivity and thermal coefficient of linear expansion of fluorite. Meas Tech 22(8):968–969
Larson AC, Von Dreele RB (2000) “General Structure Analysis System (GSAS)”, Los Alamos National Laboratory Report, LAUR 86-748
Lehmann O (1877) Ueber physikalische Isomerie. Z Kristallogr 1(4):97–131
Lengauer CL, Giester G (1995) Rietveld refinement of the solid-solution series: (Cu, Mg)SO4·H2O. Powder Diffr 10(3):189–194
Lœwel H (1855) Observations sur la sursaturation des dissolutions saline. III. Dissolutions sursaturées de sulfate de magnésie. Ann Chim Phys (3e série) 43:405–420
Lyaschenko AK, Mozhaev AP, Naumov SV, Golovchanskii AV (1983) Cryocrystallisation in water–salt systems and the structural features of highly concentrated solutions. Zh Struct Khim 24(2):48–53. doi:10.1007/BF00747381
Ma H, Bish DL, Wang H-W, Chipera SJ (2009a) Determination of the crystal structure of sanderite, MgSO4·2H2O, by X-ray powder diffraction and the charge flipping method. Am Mineral 94:622–625. doi:10.2138/am.2009.3110
Ma H, Bish DL, Wang H-W, Chipera SJ (2009b) Structure determination of the 2.5 hydrate MgSO4 phase by simulated annealing. Am Mineral 94:1071–1074. doi:10.2138/am.2009.3221
McCord TB, Hansen GB, Matson DL, Johnson TV, Crowley JK, Fanale FP, Carlson RW, Smythe WD, Martin PD, Hibbitts CA, Granahan JC, Ocampo A, the NIMS Team (1999) Hydrated salt minerals on Europa’s surface from the Galileo near-infrared mapping spectrometer (NIMS) investigation. J Geophys Res Planets 104:11827–11851. doi:10.1029/1999JE900005
McLaren White A (1933) The system ferrous sulfate–manganous sulfate–water at 0 and 25°. J Am Chem Soc 55(8):3182–3185. doi:10.1021/ja01335a019
Miles FT, Menzies AWC (1937) Solubilities of cupric sulfate and strontium chloride in deuterium water. J Am Chem Soc 59(11):2392–2395. doi:10.1021/ja01290a089
Milliken RE, Mustard JF, Poulet F, Bibring J, Langevin Y (2005) Estimating the absolute water content of Arabia Terra using Mars Express OMEGA data. AGU Fall Meeting 2005, abstract #P14A-02
Milton C, Jr Evans HT, Johnson RG (1982) Dwornikite, (Ni, Fe)SO4·H2O, a member of the kieserite group from Minasragra, Peru. Mineral Mag 46:351–355
Minguzzi C (1948) Contributi all studio della geochimica del rame. L’isoterma a 30°C. del sistema CuSO4–MgSO4–H2O. Memorie Della Societa Toscana di Science Naturali 55:26–48
Mitscherlich E (1820) Sur la relation qui existe entre la forme crystalline et les proportions chimiques. Ann Chim Phys 14:172–190
Mohammed AM, Loeffler HH, Inada Y, Tanada K, Funahashi S (2005) Quantum mechanical/molecular mechanical molecular dynamic simulation of zinc(II) ion in water. J Mol Liq 119(1–3):55–62. doi:10.1016/j.molliq.2004.10.008
Moin ST, Hofer TS, Pribil AB, Randolf BR, Rode BM (2010) A quantum mechanical charge field molecular dynamics study of Fe2+ and Fe3+ ions in aqueous solutions. Inorg Chem 49(11):5101–5106. doi:10.1021/ic1002572
Montgomery DR, Gillespie A (2005) Formation of Martian outflow channels by catastrophic dewatering of evaporite deposits. Geology 33(8):625–628. doi:10.1130/G21270.1
Oikova T, Balarew C (1974) Thermodynamic study of magnesium sulfate–ferrosulfate–water system at 25°C. CR Acad Bulg Sci 27(9):1211–1214
Oikova T, Barkov D (1979) The CoSO4–MgSO4–H2O system at 40°C. Russ J Inorg Chem 24(2):278–280
Oikova T, Balarew C, Makarow L (1976) Thermodynamical study on the systems MgSO4–CoSO4–H2O and MgSO4–ZnSO4–H2O at 25,0°C. Russ J Inorg Chem 50:347–352
Ojkova T, Makarow L, Balarew C, Miloschova M (1974) Thermodynamische Untersuchung des Systems Zinksulfat-Nickelsulfat-Wasser bei 25°C. Z Phys Chem 255:453–463
Ojkova T, Balarew C, Makarow L (1975) Untersuchung des Systems Nickelsulfat-Magnesiumsulfat-Wasser bei 25°C. Z Phys Chem 256:890–896
Pannetier G, Brégeault JM, Lecouturier C, Djega Mariadassou C (1964) Etude de la dissociation thermique des sulfates et des sulfates basiques. Quelques aspects de la dissociation thermique des sulfates de nickel, NiSO4·7H2O et NiSO4·6H2O; étude cristallographique de NiSO4·4H2O. Bull Soc Chim Franç 1964:3141–3149
Peterson RC (2003) The relationship between Cu content and distortion in the atomic structure of melanterite from the Richmond Mine, Iron Mountains, California. Can Mineral 41(4):937–949. doi:10.2113/gscanmin.41.4.937
Peterson RC (2011) Cranswickite MgSO4·4H2O, a new mineral from Calingasta, Argentina. Am Mineral 96(5–6):869–877. doi:10.2138/am.2011.3673
Peterson RC, Wang R (2006) Crystal molds on Mars: melting of a possible new mineral species to create Martian chaotic terrain. Geology 34(11):957–960. doi:10.1130/G22678A.1
Peterson RC, Roeder PL, Zhang Y (2003) The atomic structure of siderotil, (Fe, Cu)SO4·5H2O. Can Mineral 41(3):671–676. doi:10.2113/gscanmin.41.3.671
Peterson RC, Hammarstrom JM, Seal RR (2005) Alpersite (Mg,Cu)SO4·7H2O a new mineral of the melanterite group. Am Mineral 91(2–3):261–269. doi:10.2138/am.2006.1911
Peterson RC, Nelson W, Madu B, Shurvell HF (2007) Meridianiite: a new species observed on Earth and predicted to exist on Mars. Am Mineral 92(10):1756–1759. doi:10.2138/am.2007.2668
Pillay V, Gärtner RS, Himawan C, Seckler MM, Lewis AE, Witkamp G-J (2005) MgSO4 + H2O system at eutectic conditions and thermodynamic solubility products of MgSO4·12H2O(s) and MgSO4·7H2O(s). J Chem Eng Data 50(2):551–555. doi:10.1021/je0496746
Premkumar PS, Shajan XS (2010) X-ray and thermal studies on Zn x Mg1-x SO4·7H2O crystals. E-J Chem 7(S1):s121–s126
Proskurina OV, Mal’tseva ES, Rumyantsev AV, Puchkov LV (2001) A thermodynamic study of the Ni2+, Me2+//SO4 2−–H2O (Me = Mg, Zn) systems at 25°C. Russ J Phys Chem 75(3):343–348
Ptasiewicz-Bak H, Olovsson I, McIntyre GJ (1997) Charge density in orthorhombic NiSO4·7H2O at room temperature and 25 K. Acta Crystallogr B 53(3):325–336. doi:10.1107/S0108768196014061
Rammelsberg C (1854) Ueber das Verhältniss, in welchen isomorphe Körper Zusammen Krystallisieren, und den Einfluss desselben auf die Form der Krystalle. Ann Phys (2e série) 91:321–354
Redhammer GJ, Koll L, Bernroider M, Tippelt G (2007) Co2+–Cu2+ substitution in bieberite solid–solution series, (Co1-xCux)SO4.7H2O, 0.00 ≤ x ≤ 0.46: Synthesis, single-crystal structure analysis, and optical spectroscopy. Am Mineral 92(4): 532–545. doi:10.2138/am.2007.2229
Remsungnen T, Rode BM (2003) QM/MM molecular dynamics simulation of the structure of hydrated Fe(II) and Fe(III) ions. J Phys Chem A 107(13):2324–2328. doi:10.1021/jp027007i
Retgers JW (1889) Das specifische Gewicht isomorphe Mischungen. Z Phys Chem 3:497–561
Retgers JW (1895) Beitrage sur Kenntnis des Isomorphismus. Z Phys Chem 16:577–658
Rieder R, Gellerst R, Anderson RC, Bruckner J, Clark BC, Dreibus G, Economou T, Klingelhofer G, Lugmair GW, Ming DW, Squyres SW, d’Uston C, Wanke H, Yen A, Zipfel J (2004) Chemistry of rocks and soils at Meridiani Planum from the alpha particle spectrometer. Science 306:1746–1749. doi:10.1126/science.1104358
Roach LH, Mustard JF, Murchie SL, Bibring JP, Forget F, Lewis KW, Aharonson O, Vincendon M, Bishop JL (2009) Testing evidence of recent hydration state change in sulfates on Mars. J Geophys Res 114:E00D02. doi:10.1029/2008JE003245
Rode BM, Schwenk CF, Hofer TS, Randolf BR (2005) Coordination and ligand exchange dynamics of solvated metal ions. Coord Chem Rev 249(24):2993–3006. doi:10.1016/j.ccr.2005.03.032
Rüdorff F (1872) Ueber das Gefrieren der Salzlösungen. Ann Phys 221(4):599–622. doi:10.1002/andp.18722210406
Schäuffelè J-MD (1852) Ueber die mehrbasichen schwefelsauren Salze der Magnesiareihe. J Prakt Chem 55:371–379
Schwenk CF, Rode BM (2003) Extended ab initio quantum mechanical/molecular mechanical molecular dynamics simulations of hydrated Cu2+. J Chem Phys 119(18):9523–9531. doi:10.1063/1.1614224
Schwenk CF, Loeffler HH, Rode BM (2003) Structure and dynamics of metal ions in solution: QM/MM molecular dynamics simulations of Mn2+ and V2+. J Am Chem Soc 125(6):1618–1624. doi:10.1021/ja0286831
Siebke W, Spiering H, Meissner E (1983) Cooperative pseudo-Jahn-Teller effect of the Fe(H2O) 2+6 complexes in the sulfate heptahydrates. Phys Rev B 27(5):2730–2739. doi:10.1103/PhysRevB.27.2730
Simich ML, Karchenko AV, Girich TE, Saroka PI (1992) The NiSO4–ZnSO4–H2O system at the boiling point of the saturated solutions. Russ J Inorg Chem 37(10):1212–1214
Smith GS, Snyder RL (1979) F(N): a criterion for rating powder diffraction patterns and evaluating the reliability of powder-pattern indexing. J Appl Crystallogr 12(1):60–65. doi:10.1107/S002188987901178X
Smolik M (2000a) Distribution of microamounts of some M2+ ions during ZnSO4·7H2O crystallization. Can J Chem 78(7):993–1002. doi:10.1139/v00-054
Smolik M (2000b) Distribution of trace amounts of M2+ ions during crystallization of NiSO4·7H2O. Polish J Chem 74(10):1447–1461
Smolik M (2003) Cocrystallization of low amounts of M2+ ions during CoSO4·7H2O crystallization. J Chilean Chem Soc 48(3):13–18. doi:10.4067/S0717-97072003000300002
Smolik M, Lipowska B (1995) Partition of trace amounts of impurities during the crystallization of FeSO4·7H2O at 20°C. Indian J Chem A 34(3):230–234
Smolik M, Zolotajkin M (1993) Partition of trace amounts of impurities during the crystallization of CuSO4·5H2O. Polish J Chem 67(3):383–389
Smolik M, Zolotajkin M, Kluczka J (1995) Distribution of trace amounts of impurities during manganese(II) sulfate crystallization at 20° and 100°C. Polish J Chem 69(9):1322–1327
Soboleva OS (1952) Oптичecкиe cвoйcтвa cмeшaнныx кpиcтaллoв: (Mg, Ni)SO4·7H2O и (Mg, Ni)SO4·6H2O. Mineralogicheskii Sbornik- L’Vovskii Gosudarstvennyi Universitet 6:265–267
Soboleva OS (1953) Tepмичecкoe иccлeдoвaниe cмeшaнныx кpиcтaллoв ceмивoдныx cyльфaтoв мaгния и никeля. Mineralogicheskii Sbornik- L’Vovskii Gosudarstvennyi Universitet 7:247–252
Soboleva OS (1958) Piвнoвaги cиcтeмi MgSO4–NiSO4–H2O. Naukovi Zapisky L’Vivs’kogo Derzhavnogo Universytetu Imeni Ivana Franka 46:91–106
Soboleva OS (1960) Политерма paвнoвecий cиcтeмы MgSO4–NiSO4–H2O. Dokl Akad Nauk SSSR 135(1):91–93
Soma T, Kagaya H-M (1999) Thermal expansion coefficients of c-Si. In: Hull R (ed) Properties of crystalline silicon. INSPEC, The Institution of Electrical Engineers, London, pp 153–154
Squyres SW, Knoll AH (2005) Sedimentary rocks at Meridiani Planum: origin, diagenesis, and implications for life on Mars. Earth Planet Sci Lett 240(1):1–10. doi:10.1016/j.epsl.2005.09.038
Squyres SW, Grotzinger JP, Arvidson RE, Bell JF III, Calvin W, Christensen PR, Clark BC, Crisp JA, Farrand WH, Herkenhoff KE, Johnson JR, Klingelhöfer G, Knoll AH, McLennan SM, McSween HY Jr, Morris RV, Rice JW Jr, Rieder R, Soderblom LA (2004) In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science 306:1709–1714. doi:10.1126/science.1104559
Steele BT, Johnson FMG (1904) The solubility curves of the hydrates of nickel sulphate. J Chem Soc Trans 85:113–120. doi:10.1039/ct9048500113
Steiger M, Linnow K, Ehrhardt D, Rohde M (2011) Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4–H2O and Na+–Mg2+–Cl−–SO 2−4 –H2O systems with implications for Mars. Geochim Cosmochim Acta 75(12):3600–3626. doi:10.1016/j.gca.2011.03.038
Strunz H (1957) Mineralogische Tabellen, 3rd edn. Akademische Verlagsgesellschaft, Leipzig
Takegami S (1922) On the octahydrate of magnesium sulphate. Mem Coll Sci Kyoto Imperial Univ 5:191–199
Theivanayagam M, Mahadevan C (2001) Lattice variations and thermal parameters of Ni x Mg1− x SO4·7H2O single crystals. Bull Mat Sci 24(5):441–444. doi:10.1007/BF02706713
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Appl Crystallogr 34(2):210–213. doi:10.1107/S0021889801002242
Tongraar A, Bernd RM (2005) Structural arrangement and dynamics of the hydrated Mg2+: an ab initio QM/MM molecular dynamics simulation. Chem Phys Lett 409(4–6):304–309. doi:10.1016/j.cplett.2005.04.062
Trendafelov D, Balarew C (1968) Untersuchung der Verteilung der Kobaltionen in verschiedenen Zinksulfathydraten. Comm Bulg Acad Sci Dept Chem 1:73–80
Viola C (1923) Über bestimmte Mischkristalle. Z Kristallogr 58:583–595
Volger GHO (1855) Tauriszit, ein neue Subgenus des Eisen-Vitriols. Neues Jb Miner Geogn Geol Petrefakt 23:152–158
Wang A, Haskin LA, Squyres SW, Jolliff BL, Crumpler L, Gellert R, Schroder C, Herkenhoff K, Hurowitz J, Tosca NJ, Farrand WH, Anderson R, Knudson AT (2006) Sulfate deposition in subsurface regolith in Gusev crater, Mars. J Geophys Res 111:E02S17. doi:10.1029/2005JE002513
Wang A, Freeman JJ, Chou I-M, Joliff BL (2011) Stability of Mg-sulfates at −10°C and the rates of dehydration/rehydration processes under conditions relevant to Mars. J Geophys Res 116:E120006. doi:10.1029/2011JE003818
Wood IG, Hughes N, Browning F, Fortes AD (2012) A compact, transportable, thermoelectrically-cooled cold stage for reflection geometry X-ray powder diffraction. J Appl Crystallogr (in press)
Zalkin A, Ruben H, Templeton DH (1964) The crystal structure and hydrogen bonding of magnesium sulfate hexahydrate. Acta Crystallogr 17(3):235–240. doi:10.1107/S0365110X64000603
Zhelnin BI, Gorshtein GI (1971) Equilibrium in MnSO4–MgSO4–H2O system at 25° and 100°C. Russ J Inorg Chem 16(11):1668–1670
Acknowledgments
The authors thank Neil Hughes for assistance with the design and construction of the Peltier cold stage, Kevin Knight for the use of the cryogenic pestle and mortar, and two anonymous referees for providing the most thorough and constructive reviews possible. This work was supported in part by Master’s student funds from the UCL Department of Earth Sciences (F. Browning), and in part by the Science and Technology Facilities Council, Fellowship number PP/E006515/1 (A. D. Fortes).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fortes, A.D., Browning, F. & Wood, I.G. Cation substitution in synthetic meridianiite (MgSO4·11H2O) I: X-ray powder diffraction analysis of quenched polycrystalline aggregates. Phys Chem Minerals 39, 419–441 (2012). https://doi.org/10.1007/s00269-012-0497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-012-0497-9