Abstract
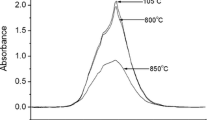
Dehydration behavior of muscovite flake was investigated at 760–860°C by using in situ high-temperature IR microspectroscopy for the OH absorption band around 3,620 cm−1. Isothermal kinetic heating experiments at each temperature gave detailed decrease curves of the OH band area with time. These curves have been simulated by the first and second order reactions or mono- and two-dimensional diffusion processes. The mono-dimensional diffusion was found to give the best fit to the experimental data and apparent diffusion coefficients D were determined at 760–860°C with the activation energy of 290 ± 20 kJ/mol. The apparent diffusion coefficients D varied with the sample thickness L. This variation can be explained by an m layers model with a unit length of L′ with a constant diffusion coefficient D′. Therefore, the dehydration process might be rate-limited by mono-dimensional diffusion through tetrahedral silicate sheet perpendicular to (001) planes of muscovite with a unit length of L′.













Similar content being viewed by others
References
Abbott RN Jr (1994) Energy calculations bearing on the dehydroxylation of muscovite. Can Mineral 32:87–92
Aines RD, Rossman GR (1985) The high temperature behavior of trace hydrous components in silicate minerals. Am Mineral 70:1169–1179
Bailey SW (1984) Crystal chemistry of the true micas. In: Bailey SW (ed) Micas. Reviews in Mineralogy, vol 13. Mineralogical Society of America, pp 13–16
Brindley GW, Brown G (1980) Crystal structures of clay minerals and their X-ray identification. Mineralogical Society, London
Carslaw HS, Jaeger JC (1959) Conduction of heat in solids, vol 2. Oxford University Press, London
Eberhart JP (1963) Etude des transformations du mica muscovite par chauffage entre 700 et 1200°C. Bull Soc Fr Minéral Cristallogr 86:213–251
Elphick SC, Graham CM, Dennis PF (1988) An ion microprobe study of anhydrous oxygen diffusion in anorthite: a comparison with hydrothermal data and some geological implications. Contrib Mineral Petrol 100:490–495. doi:10.1007/BF00371378
Farver JR, Yund RA (1991) Oxygen diffusion in quartz: dependence on temperature and water fugacity. Chem Geol 90:55–70. doi:10.1016/0009-2541(91)90033-N
Fortier SM, Giletti BJ (1991) Volume self-diffusion of oxygen in biotite, muscovite and phlogopite micas. Geochim Cosmochim Acta 55:1319–1330. doi:10.1016/0016-7037(91)90310-2
Fyfe WS, Price NJ, Thompson AB (1978) Fluids in the earth’s crust. Elsevier, Amsterdam
Gaines GL Jr, Vedder W (1964) Dehydroxylation of muscovite. Nature 201:495. doi:10.1038/201495a0
Giletti BJ, Yund RA (1984) Oxygen diffusion in quartz. J Geophys Res 89:4039–4046. doi:10.1029/JB089iB06p04039
Gridi-Bennadji F, Blanchart P (2007) Dehydroxylation kinetic and exfoliation of large muscovite flakes. J Therm Anal Calorim 90:747–753. doi:10.1007/s10973-006-7888-4
Guggenheim S, Chang Y-H, Koster van Groos AF (1987) Muscovite dehydration: high temperature studies. Am Mineral 72:537–550
Hanykyr V, Ederova J, Travnicek Z, Srank J (1985) Isothermal dehydroxylation of muscovite mica. Thermochim Acta 93:517–520. doi:10.1016/0040-6031(85)85130-3
Holt JB, Cutler IB, Wadsworth ME (1958) Rate of thermal dehydration of muscovite. J Am Ceram Soc 41:242–246. doi:10.1111/j.1151-2916.1958.tb13548.x
Holt JB, Cutler IB, Wadsworth ME, Klein C, Hurlbut CS Jr (1964) Kinetics of the thermal dehydration of hydrous silicates. Clays Clay Miner 12:55–67. doi:10.1346/CCMN.1963.0120109
Kitamura M, Kondoh S, Morimoto N, Miller GH, Rossman GR, Putnis A (1987) Planar OH-bearing defects in mantle olivine. Nature 328:143–145. doi:10.1038/328143a0
Klein C, Hurlbut CS Jr (1993) Manual of mineralogy, 21st edition edn. Wiley and Sons, New York, pp 515–517 (after James D. Dana)
Kodama H, Brydon JE (1968) Dehydration of microcrystalline muscovite. Trans Faraday Soc 63:3112–3119
Kogure T, Nespolo M (1999) A TEM study of long-period mica polytypes: determination of the stacking sequence of oxybiotite by means of atomic resolution images and periodic intensity distribution (PID). Acta Crystallogr B 55:507–516. doi:10.1107/S0108768199003845
Mazzucato E, Artioli G, Gualtieri A (1999) High temperature dehydroxylation of muscovite-2 M (1): a kinetic study by in situ XRPD. Phys Chem Miner 26:375–381. doi:10.1007/s002690050197
Okumura S, Nakashima S (2004) Water diffusivity in rhyolitic glasses as determined by in situ IR spectroscopy. Phys Chem Miner 31:183–189. doi:10.1007/s00269-004-0383-1
Okumura S, Nakashima S (2005) Molar absorptivities of OH and H2O in rhyolitic glass at room temperature and at 400–600°C. Am Mineral 90:441–447. doi:10.2138/am.2005.1740
Okumura S, Nakashima S (2006) Water diffusion in basaltic to dacitic glasses. Chem Geol 227:70–82. doi:10.1016/j.chemgeo.2005.09.009
Okumura S, Nakamura M, Nakashima S (2003) Determination of molar absorptivity of IR fundamental OH-stretching vibration in rhyolitic glasses. Am Mineral 88:1657–1662
Rossman GR (1984) Spectroscopy of micas. In: Bailey SW (ed) Micas. Reviews in Mineralogy, vol 13. Mineral Society of America, Washington DC, pp 145–181
Rouxhet PG (1970) Kinetics of dehydroxylation and of OH–OD exchange in macrocrystalline micas. Am Mineral 55:841–853
Sabatier MG (1955) Les transformations du mica muscovite aux environs de 700°C. Bull Groupe Fr Argiles 6:35–39
Shishelova TI, Metslk MS, Sokolov KY (1974) Changes in the IR spectra of micas on heating. Appl Spectrosc 20:784–786. doi:10.1007/BF00614158
Toraya H (1981) Distortions of octahedra and octahedral sheets in 1 M micas and the relation to their stability. Z Kristallogr 144:42–52
Udagawa S, Urabe K, Ikawa H, Katoo K, Hasu H (1973) The study on thermal transformations of muscovite by Weissenberg camera with high temperature apparatus. J Clay Sci Soc Jpn 13:131–138
Udagawa S, Urabe K, Hasu H (1974) The crystal structure of muscovite dehydroxylate. Jpn Assoc Miner Petrol Econ Geol 69:381–389
Acknowledgments
We are grateful to SII-NT for their kind assistance in thermogravimetry of the samples. We thank Ms. M. Yoshizaki of Tokyo Institute of Technology for her assistance of the EPMA analyses. We also thank Dr. M. Katsura, Mr. Y. Kirino and Dr. T. Yokoyama of Osaka University for their helpful supports in the data fitting procedures and constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tokiwai, K., Nakashima, S. Dehydration kinetics of muscovite by in situ infrared microspectroscopy. Phys Chem Minerals 37, 91–101 (2010). https://doi.org/10.1007/s00269-009-0313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-009-0313-3