Abstract
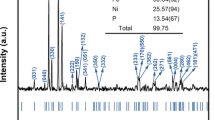
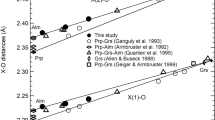
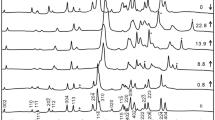
In situ X-ray diffraction measurements of Fe- and Al-bearing MgSiO3-rich perovskite (FeAl-Pv), which was synthesized from a natural orthopyroxene, were performed at pressures of 19–32 GPa and temperatures of 300–1,500 K using a combination of a Kawai-type apparatus with eight sintered-diamond anvils and synchrotron radiation. Two runs were performed using a high-pressure cell with two sample chambers, and both MgSiO3 perovskite (Mg-Pv) and FeAl-Pv were synthesized simultaneously in the same cell. Thus we were able to measure specific volumes (V/V 0) of Mg-Pv and FeAl-Pv at the same P−T conditions. At all the measurement conditions, values of the specific volume of FeAl-Pv are consistent with those of Mg-Pv within 2 Standard Deviation, strongly suggesting that effect of incorporation of iron and aluminum on the thermoelastic properties of magnesium silicate perovskite is undetectable in this composition, pressure, and temperature range. Two additional runs were performed using a high-pressure cell that has one sample chamber and unit-cell volumes of FeAl-Pv were measured at pressures and temperatures up to 32 GPa and 1,500 K, respectively. All the unit-cell volume data of FeAl-Pv perovskite were fitted to the high temperature Birch–Murnaghan equation of state and a complete set of thermoelastic parameters of this perovskite was determined with an assumption of K′ 300,0 = 4. The determined parameters are K 300,0 = 243(3) GPa, (∂K T,0/∂T) P = −0.030(8) GPa/K, a 0 = 2.78(18) × 10−5 K−1, and b 0 = 0.88(28) × 10−8 K−2, where a 0 and b 0 are the coefficients of the following expression describing the zero-pressure thermal expansion: α T,0 = a 0 + b 0 T. The equation-of-state parameters of FeAl-Pv are in good agreement with those of MgSiO3 perovskite at the conditions corresponding to the uppermost part of the lower mantle.







Similar content being viewed by others
References
Anderson OL, Isaak DG, Yamamoto S (1989) Anharmonicity and the equation of state for gold. J Appl Phys 65:1534–1543
Andrault D, Bolfan-Casanova N, Guignot N (2001) Equation of state of lower mantle (Al,Fe)-MgSiO3 perovskite. Earth Planet Sci Lett 193:501–508
Becerro AI, McCammon C, Langenhorst F, Seifert F, Angel R (1999) Oxygen vacancy ordering in CaTiO3–CaFeO2.5 perovskites: from isolated defects to infinite sheets. Phase trans 69:133–146
Daniel I, Cardon H, Fiquet G, Guyot F, Mezouar M (2001) Equation of state of Al-bearing perovskite to lower mantle pressure conditions. Geophys Res Lett 28:3789–3792
Fei Y, Mao HK, Shu J, Hu J (1992) P–V–T equation of state of magnesiowüstite (Mg0.6,Fe0.4)O. Phys Chem Miner 18:416–422
Fiquet G, Andrault D, Dewaele A, Charpin T, Kunz M, Haüsermann D (1998) P–V–T equation of state of MgSiO3 perovskite. Phys Earth Planet Inter 105:21–31
Funamori N, Yagi T (1993) High pressure and high temperature in situ X-ray observation of MgSiO3 perovskite under lower mantle conditions. Geophys Res Lett 20:387–390
Funamori N, Yagi T, Utsumi W, Kondo T, Uchida T, Funamori M (1996) Thermoelastic properties of MgSiO3 perovskite determined by in situ X ray observations up to 30 GPa and 2,000 K. J Geophys Res 101:8257–8269
Harte B, Harris JW (1994) Lower mantle mineral associations preserved in diamonds. Mineral Mag A 58:384–385
Hayman PC, Kopylova MG, Kaminsky FV (2005) Lower mantle diamonds from Rio Soriso (Juina area, Mato Grosso, Brazil). Contrib Miner Petrol 149:430–445
Hazen RM (1993) Comparative compressibilities of silicate spinels: anomalous behavior of (Mg,Fe)2SiO4. Science 259:206–209
Hazen RM, Finger W (1989) High-pressure crystal chemistry of andradite and pyrope: revised procedures for high-pressure diffraction experiments. Am Miner 74:352–359
Heinz DL, Jeanloz R (1984) The equation of state of the gold calibration standard. J Appl Phys 55:885–893
Irifune T (1994) Absence of aluminous phase in the upper part of the Earth’s lower mantle. Nature 370:131–133
Kato T, Ohtani E, Morishima H, Yamazaki D, Suzuki A, Suto M, Kubo T, Kikegawa T, Shimomura O (1995) In situ X-ray observation of high-pressure phase transitions of MgSiO3 and thermal expansion of MgSiO3 perovskite at 25 GPa by double-stage multianvil system. J Geophys Res 100:20475–20481
Kubo A, Yagi T, Ono S, Akaogi M (2000) Compressibility of Mg0.9Al0.2Si0.9O3 perovskite. In: Proceeding of the Japan academy, pp. 103–107
Mao HK, Hemley RJ, Fei Y, Shu F, Chen LC, Jephcoat AP, Wu Y, Bassett WA (1991) Effect of pressure, temperature, and composition on lattice parameters and density of (Fe, Mg)SiO3-perovskites to 30 GPa. J Geophys Res 96:8069–8079
McCammon C (1997) Perovskite as a possible sink for ferric iron in the lower mantle. Nature 387:694–696
McCammon C, Hutchison M, Harris J (1997) Ferric iron content of mineral inclusions in diamonds from São Luiz: a view in the lower mantle. Science 278:434–436
Morishima H, Ohtani E, Kato T, Shimomura O, Kikegawa T (1994) Thermal expansion of MgSiO3 perovskite at 20.5 GPa. Geophys Res Lett 21:899–902
Nishiyama N, Yagi T (2003) Phase relation and mineral chemistry in pyrolite to 2,200°C under the lower mantle pressures and implications for dynamics of mantle plumes. J Geophys Res 108 Doi:10.1029/2002JB002216
Richmond NC, Brodholt JP (1998) Calculated role of aluminum in the incorporation of ferric iron into magnesium silicate perovskite. Am Miner 83:947–951
Ringwood AE (1966) The chemical composition and origin of the earth. In Hurley P (ed): Advances in Earth Sciences. MIT Press, Cambridge, pp 287–356
Ross NL, Hazen RM (1989) Single crystal X-ray diffraction study of MgSiO3 perovskite from 77 to 400 K. Phys Chem Miner 16:415–420
Ross NL, Angel RJ, Seifert F (2002) Compressibility of brownmillerite (Ca2Fe2O5): effect of vacancies on the elastic properties of perovskites. Phys Earth Planet Inter 129:145–151
Saxena SK, Zhang J (1990) Thermochemical and pressure–volume–temperature systematics of data on solids, example: tungsten and MgO. Phys Chem Miner 17:45–51
Shim SH, Duffy TS, Takemura K (2002) Equation of state of gold and its application to the phase boundaries near 660 km depth in the Earth’s mantle. Earth Planet Sci Lett 203:729–739
Sinogeikin S, Zhang J, Bass JD (2004) Elasticity of single crystal and polycrystalline MgSiO3 perovskite by Brillouin spectroscopy. Geophys Res Lett 31 DOI 10.1029/2004GL019559
Stebbins JF, Kojitani H, Akaogi M, Navrotsky A (2003) Aluminum substitution in MgSiO3 perovskite: investigation of multiple mechanisms by 27Al NMR. Am Miner 88:1161–1164
Takei H, Hosoya S, Ozima M (1984) Synthsis of large single crystals of silicates and titanates. In: Sunagawa I (ed) Materials science of the Earth’s interior. TERRAPAB, Tokyo, pp 107–130
Takemura K (2001) Evaluation of the hydrostaticity of a helium-pressure medium with powder x-ray diffraction techniques. J Appl Phys 89:662–668
van Thiel M, Kusubov AS, Mitchell AC (1967) Compendium of shock wave data, Technical Report UCRL-50108, Lawrence Radiation Laboratory, Livermore
Utsumi W, Funamori N, Yagi T, Ito E, Kikegawa T, Shimomura O (1995) Thermal expansivity of MgSiO3 perovskite under high pressures up to 20 GPa. Geophys Res Lett 22:1005–1008
Vanpeteghem CB, Angel RJ, Ross NL, Jacobsen SD, Dobson DP, Litasov KD, Ohtani E (2006) Al, Fe substitution in the MgSiO3 perovskite structure: a single-crystal X-ray diffraction study. Phys Earth Planet Inter 155:96–103
Wang Y, Weidner DJ, Liebermann RC, Liu X, Ko J, Vaughan MT, Zhao Y, Yeganeh-Haeri A, Pacalo REG (1991) Phase transition and thermal expansion of MgSiO3 perovskite. Science 251:410–413
Wang Y, Weidner DJ, Liebermann RC, Zhao Y (1994) P–V–T equation of state of (Mg, Fe)SiO3 pervskite: constraints on composition of the lower mantle. Phys Earth Planet Inter 83:13–40
Wood BJ, Rubie DC (1996) The effect of alumina on phase transformations at the 660-km discontinuity from Fe-Mg partitioning experiments. Nature 273:1522–1524
Yagi T, Okabe K, Nishiyama N, Kubo A, Kigegawa T (2004) Complicated effects of aluminum on the compressibility of silicate perovskite. Phys Earth Planet Inter 143–144:81–89
Zhang J, Weidner DJ, (1999) Thermal equation of state of alumium-enriched silicate perovskite. Science 284:782–784
Acknowledgments
The authors thank J. Ando for providing natural orthopyroxene from San-Carlos as a starting material. We also thank T. Kondo for providing heater material. We thank M. Akaogi, Y. Wang, and H. Ohfuji for constructive comments and discussion. We also thank Y. Tange for help in the in-situ X-ray observations at Photon Factory, KEK. We are grateful to F. Sakai and M. Otsuki for their helps in chemical composition analyses at MDCL, ISSP and ERI, respectively, the University of Tokyo. We thank N. Funamori and an anonymous reviewer for constructive reviews. This work was performed at BL-14C under the approval of the Program Advisory Committee. The present study is partly supported by the Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists to N. N.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishiyama, N., Yagi, T., Ono, S. et al. Effect of incorporation of iron and aluminum on the thermoelastic properties of magnesium silicate perovskite. Phys Chem Minerals 34, 131–143 (2007). https://doi.org/10.1007/s00269-006-0134-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0134-6