Abstract
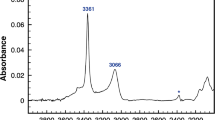
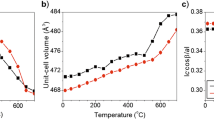
The solubility and incorporation mechanisms of hydrogen in synthetic stishovite as a function of Al2O3 content have been investigated. Mechanisms for H incorporation in stishovite are more complex than previously thought. Most H in stishovite is incorporated via the Smyth et al. (Am Mineral 80:454–456, 1995) model, where H docks close to one of the shared O–O edges, giving rise to an OH stretching band in infrared (IR) spectra at 3,111–3,117 cm−1. However, careful examination of IR spectra from Al-stishovite reveals the presence of an additional OH band at 3,157–3,170 cm−1. All H is present on one site, with interstitial H both coupled to Al3+ substitutional defects on adjacent octahedral (Si4+) sites, and decoupled from other defects, giving rise to two distinct absorption bands. Trends in IR data as a function of composition are consistent with a change in Al incorporation mechanism in stishovite, with Al3+ substitution for Si4+ charge-balanced by oxygen vacancies at low bulk Al2O3 contents, and coupled substitution of Al3+ onto octahedral (Si4+) and interstitial sites at high bulk Al2O3 contents. Trends in OH stretching frequencies as a function of Al2O3 content suggest that any such change in Al incorporation mechanism could alter the effect that Al incorporation has on the compressibility of stishovite, as noted by Ono et al. (Am Mineral 87:1486–1489, 2002).



Similar content being viewed by others
References
Asimov P, Stein L, Mosenfelder J, Rossman G (2006) Quantitative polarized infrared analysis of trace OH in populations of randomly oriented mineral grains. Am Mineral 86:147–158
Bromiley GD, Hilairet N (2005) An investigation of hydrogen and minor element incorporation in synthetic rutile. Mineral Mag 69(3):345–358
Bromiley GD, Keppler H (2004) An experimental investigation of hydroxyl solubility in jadeite and Na-rich pyroxenes. Contrib Mineral Petrol 147:189–200
Bromiley GD, Shiryaev AA (2006) Neutron irradiation and post-irradiation annealing of rutile (TiO2−x ): effect on hydrogen incorporation and optical absorption. Phys Chem Miner 33:426–434
Bromiley GD, Hilairet N, McCammon C (2004) Solubility of hydrogen and ferric iron in rutile and TiO2 (II): implications for phase assemblages during ultrahigh-pressure metamorphism and for the stability of silica polymorphs in the lower mantle. Geophys Res Lett 31:L04610
Chung J, Kagi H (2002) High concentration of water in stishovite in the MORB system. Geophys Res Lett 29(21). DOI 10.1029/2002GL015579
Demouchy S, Deloule E, Frost D, Keppler H (2005) Pressure and temperature-dependence of water solubility in Fe-free wadsleyite. Am Mineral 90:1084–1091
Gesenhues U, Rentschler T (1999) Crystal growth and defect structure of Al3+-doped rutile. J Solid State Chem 143:210–218
Gibbs G, Cox D, Ross N (2004) A modeling of the structure and favourable H-docking sites and defects for the high-pressure silica polymorph stishovite. Phys Chem Miner 31:232–239
Hirose K, Takafuji N, Sata N, Ohishi Y (2005) Phase transition and density of subducted MORB crust in the lower mantle. Earth Planet Sci Lett 23(1–2):239–251
Khomenko V, Langer K, Rager H, Fett A (1998) Electronic absorption by Ti3+ ions and electronic delocalization in synthetic blue rutile. Phys Chem Miner 25:338–346
Kröger FA, Vink HJ (1956) Relations between the concentrations of imperfections in crystalline solids. In: Seitz F, Turnball D (eds) Solid state physics: advances and applications, vol 3. Academic, New York, pp. 307–435
Libowitzky E (1999) Correlation of O–H stretching frequencies and O–H···O hydrogen bond lengths in minerals. Monatsh Chem 130(8):1047–1059
Libowitzky E, Rossman G (1996) Principles of quantitative absorbance measurements in anisotropic crystals. Phys Chem Miner 23:319–327
Mierdel K, Keppler H (2004) The temperature dependence of water solubility in enstatite. Contrib Mineral Petrol 148(3):305–311
Ono S (1999) High temperature stability limit of phase egg, AlSiO3(OH). Contrib Mineral Petrol 137:83–89
Ono S, Ito E, Katsura T (2001) Mineralogy of subducted basaltic crust (MORB) from 25 to 37 GPa, and chemical heterogeneity of the lower mantle. Earth Planet Sci Lett 190:57–63
Ono S, Suto T, Hirose K, Kuwayama Y, Komabayashi T, Kikegawa T (2002) Equation of state of Al-bearing stishovite to 40 GPa at 300 K. Am Mineral 87(10):1486–1489
Panero W, Benedetti L, Jeanloz R (2003) Transport of water into the lower mantle: role of stishovite. J Geophy Res 108(B1). DOI 10.1029/2002JB002053
Pawley A, McMillan P, Holloway J (1993) Hydrogen in stishovite, with implications for mantle water-content. Science 261(5124):1024–1026
Smyth J, Bell D, Rossman G (1991) Incorporation of hydroxyl in upper-mantle clinopyroxenes. Nature 351:732–735
Smyth J, Swope R, Pawley A (1995) H in rutile-type compounds: II. Crystal chemistry of Al substitution in H-bearing stishovite. Am Mineral 80:454–456
Swope R, Smyth J, Larson A (1995) H in rutile compounds: I. Single-crystal neutron and X-ray diffraction study of H in rutile. Am Mineral 80:448–453
Vlassopoulos D, Rossman G, Haggerty S (1993) Coupled substitution of H and minor elements in rutile and the implications of high OH contents in Nb- and Cr-rich rutile from the upper mantle. Am Mineral 78:1181–1191
Acknowledgments
GDB thanks Dan Frost for help with MA experiments and Hans Keppler for providing additional access to IR facilities at the BGI. This manuscript was improved following comments by David F. Cox and an anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bromiley, G.D., Bromiley, F.A. & Bromiley, D.W. On the mechanisms for H and Al incorporation in stishovite. Phys Chem Minerals 33, 613–621 (2006). https://doi.org/10.1007/s00269-006-0107-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0107-9