Abstract
Background
Our aim was to determine whether substitution of goal-directed fluid therapy (GDT) (perioperative fluid administration) for traditional therapy to manage elderly patients with coronary heart disease scheduled for gastrointestinal (GI) surgery was advantageous. We determined if it would reduce cardiac complications and shorten time to recovery and discharge.
Methods
Altogether, 60 of these elderly patients were randomized into GDT (n = 30) and control (n = 30) groups. In the GDT group, fluid management was carried out under guidance of hemodynamic status indicators. Types and quantities of fluids administered, blood loss, intraoperative urine output, time of extubation, intensive care unit (ICU) stay, hospital stay, postoperative adverse cardiac events, and GI complications were recorded.
Results
Total fluids infused were 2,910 ± 645 ml (GDT group) and 3,640 ± 771 ml (control group) (p < 0.05). Numbers of adverse cardiac events in the two groups were not significantly different (p = 0.121). Return of GI function was significantly faster in the GDT group (p < 0.001). Median ICU stay was 32.5 h in the GDT group and 47.5 h in the control group (p < 0.001). Median hospital stay was 18 days in the GDT group and 22 days in the control group (p < 0.001).
Conclusions
GDT was associated with shorter ICU stay and time to discharge and faster return of GI function compared to traditional fluid therapy. The number of adverse cardiac events was similar in the two groups.
Similar content being viewed by others
Introduction
During surgery, oxygen delivery and organ perfusion are often impaired owing to changes in hemodynamics and the metabolic needs of various organ systems. This situation is especially evident in the gastrointestinal (GI) system in patients undergoing major surgical procedures, particularly cardiac surgery [1, 2]. Hypoperfusion of the GI organs occurs in up to 63 % of major surgical procedures and is associated with increased morbidity and length of hospital stay [1]. In patients undergoing major operative procedures, traditional fluid therapy does not take into account the surgery type, anesthesia method, or preoperative fluid status of the patient [3].
The use of goal-directed fluid therapy (GDT) is currently a growing trend for the management of perioperative fluid therapy during major surgical procedures [4–7]. In essence, GDT uses indices such as cardiac output or the arterial pressure waveform to guide perioperative fluid management [4–9]. Recent studies have shown that GDT can reduce morbidity and length of hospital stay in certain high-risk surgical patients [10–13]. Although GDT has been shown to be beneficial in certain situations, the patient populations likely to benefit the most have yet to be clearly identified. In young, otherwise healthy patients, GDT has been associated with poorer outcomes than traditional fluid management [12, 14]. In particular, there have been no clinical studies of the value of GDT for a particularly high-risk group: elderly patients with coronary heart disease undergoing GI surgery.
We hypothesized that GDT would decrease the incidence of cardiovascular complications and shorten the time to recovery and discharge in this particular patient group. The current randomized study compared GDT and conventional fluid therapy in regard to cardiovascular complications and clinical outcome for these patients.
Methods
Patients
The Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University approved this study on October 20, 2010. It is registered in the World Health Organization’s (WHO) International Clinical Trials Registry Platform (Chinese Clinical Trial Registry: http://www.chictr.org/cn/ Registration No. ChiCTR-TRC-11001405). All patients provided written informed consent after a detailed explanation of the study, and all patients took part in this study voluntarily.
A total of 60 elderly patients with coronary heart disease undergoing GI surgery in our hospital between March 1999 and March 2011 were included in the current study. Patients were randomized to receive GDT or routine fluid therapy using a random number generator (odd for GDT, even for routine therapy). Numbers were placed in sealed envelopes and opened by the anesthesiologist in charge just prior to induction of anesthesia.
Inclusion criteria were (1) 60–80 years old; (2) had undergong coronary arteriography for identification and stratification of cardiac disease; (3) scheduled for moderate- to high-risk elective GI surgery; (4) met the WHO diagnostic criteria for coronary heart disease; (5) judged able to tolerate the fluid therapy and 3–5 h of operating time; (6) New York Heart Association (NYHA) classification II–III; (7) body mass index (BMI) 18–24 kg/m2; (8) anticipated intraoperative blood loss was <600 ml; (9) normal renal and liver function; (10) provided informed consent before surgery.
Exclusion criteria were: (1) scheduled for emergency or low-risk surgery; (2) American Society of Anesthesiologists (ASA) grade >III; (3) received prior fluid therapy (>2,500 ml/day over the 48 h before the surgery); (4) presence of congenital heart disease, cardiomyopathy, rheumatic heart disease, or pulmonary heart disease; (5) preoperative use of vasoactive drugs (e.g. digoxin, nitroglycerine, nifedipine) for ≥3 months; (6) preoperative or intraoperative administration of diuretics; (7) preoperative acid–base imbalance or electrolyte (Na, K, Ca, Mg) imbalance; (8) difficulty (or contraindication to) placing a central venous catheter; (9) inability to cooperate (e.g. mental disorder, disturbance of consciousness, mental retardation); (10) presence of blood-borne infectious disease (e.g. hepatitis B, hepatitis C, syphilis, acquired immunodeficiency syndrome); (11) had undergone surgery twice after admission. Patients were also excluded if the preoperative evaluation of fluid status indicated that they were dehydrated.
Monitoring
After admission, their electrocardiogram (ECG), heart rate, saturation of peripheral oxygen (SpO2), Pleth Variability Index (PVI), mean arterial pressure (MAP), and temperature were monitored with the Philips IntelliVue Patient Monitor (Philips, Eindhoven, The Netherlands). The depth of sedation was monitored with the Masimo Radical 7 Patient monitor (Masimo, Irvine, CA, USA). The electroencephalography (EEG) monitor used was from Narcotrend (Hannover, Germany). Catheterization of the left radial artery and right internal jugular vein was performed under local anesthesia. The left radial artery was connected to the Vigileo/FloTrac system (Edwards Lifesciences, Irvine, CA, USA) for monitoring cardiac output, stroke volume, stroke volume variation (SVV), stroke volume index (SVI), cardiac index, systemic vascular resistance (SVR), and the SVR index (SVRI). The right internal jugular vein was connected to the monitor for observing central venous pressure. Urine volume, recorded to guide fluid therapy, was maintained at >0.5 ml/kg/h.
Induction and maintenance of anesthesia
All patients in both groups received general anesthesia, which was induced with intravenous fentanyl citrate (4–6 mg/kg), vecuronium bromide (0.1 mg/kg), and midazolam (0.05 mg/kg). Endotracheal intubation was carried out after the depth of anesthesia reached DE2-E1 (corresponding to the bispectral index 45–50). The tidal volume was maintained at 8–10 ml/kg, the respiratory rate at 10–14 breaths/min, the inspiratory/expiratory ratio at 1:2, and PaCO2 at 35–45 mmHg. For anesthesia maintenance, the amount of intraoperative midazolam, vecuronium, fentanyl, and sevoflurane administered was guided by the Narcotrend and TOF-WATCH (Bluestar Enterprises, Omaha, BE, USA) monitors to maintain the depth of anesthesia at D2-E1.
Preoperative evaluation of fluid status and methods of fluid infusion
Evaluation of fluid status was carried out before fluid therapy in both groups. Evaluation consisted of: (1) observing the patient’s mental condition, skin elasticity, duration of fasting, and loss of body fluid; (2) measuring the blood pressure (BP) and heart rate (HR); and (3) determining electrolyte levels and performing liver and kidney function tests. Based on the above data, dehydrated patients were excluded from the study because the management and outcome of these patients might be affected by the patient’s preoperative condition instead of by the method of fluid administration.
Control group
For the control group, all fluid administration followed standard guidelines [15]. In this group, 5–7 ml/kg (depending on the preoperative evaluation of the fluid status) of balanced salt solution (BSS) was infused during the 30 min previous to surgery. For maintenance, a basal amount determined using the 4/2/1 rule (body weight 0–10 kg, 40 ml/h; body weight 11–20 kg, 60 ml/h; body weight >21 kg, 65 ml/h) plus an additional amount to compensate for redistribution of fluid due to surgery (2 ml/kg/h for a small incision, 4–6 ml/kg/h for a large incision) of BSS (without glucose) was infused. Ascitic fluid drainage was replaced with BSS, and blood loss was replaced with an equal volume of hydroxyethyl starch (HES 130/0.4; Fresenius Kabi, Bad Homburg, Germany). If the blood loss was >15 % of the blood volume, it was given with added packed red blood cells (RBCs) [15]. Acid–base imbalances and electrolyte disturbances were managed according to the results of intraoperative blood gas analyses.
GDT group
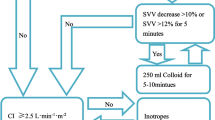
In the GDT group, patients also received BSS (5–7 ml/kg depending on the preoperative evaluation of the fluid status) infused during the 30 min prior to surgery, and GDT was used intraoperatively and throughout the first 24 h postoperatively. During anesthesia, if the cardiac index (CI) and SVI were both low (<2.5 l/min/m2 and <35 ml/min2, respectively) and the stroke volume variation was high (>12 %), a bolus of BSS (500 ml) was administered. If that failed, an added bolus (250 ml) of colloid solution (HES 130/0.4) was given to increase the SVI to >35. The maximum amount of colloid that could be given was 30 ml/kg/day. If administration of fluid failed, dopamine or norepinephrine were used to maintain the CI >2.5 and MAP >65.9.
If the amplitude of changes in heart rate and MAP were >30 % higher or lower than the basal values over a period of >10 min, if recovery to basal values could not be achieved after fluid infusion, or if hemodynamic instability was present, intravenous nitroglycerin or ephedrine to correct the situation was slowly administered (5–10 μg/min). In the GDT group, GDT was continued for the first 24 h after surgery. The summarized guide is shown in Fig. 1.
Outcome measures
Outcome assessors were blinded to the grouping of the patients. The incidence of adverse cardiac events was determined using the following criteria. Myocardial ischemia was defined as flat or down-sloping ST segment depression ≥1 mm with or without T wave inversion. Acute myocardial infarction was defined as precordial pain for >30 min, a Q wave on the electrocardiogram (ECG), ST segment depression or concave upward ST segment elevation, and cTn I > 3.1 g/l. A 12-lead ECG was obtained once a day until discharge. cTnI determinations were performed once a day for the first three postoperative days and then once every 3 days until discharge. Serious arrhythmias were defined as atrial fibrillation with significant symptoms or affecting hemodynamics and requiring medication, paroxysmal supraventricular tachycardia, and premature ventricular contraction. Congestive heart failure was defined as shortness of breath, jugular vein distention, gallop rhythm, and pulmonary edema shown by chest radiography. Cardiac death was defined as death due to myocardial infarction, heart failure, arrhythmias, or other cardiac causes.
Postoperative GI dysfunction included indigestion, severe vomiting, diarrhea, GI bleeding, and acute intestinal obstruction. Other outcome measures included time to extubation, length of intensive care unit (ICU) stay, GI recovery, and length of hospitalization.
Respiratory and cardiovascular indicators were recorded at entry to the operating room, after induction of anesthesia, at the beginning of surgery, 1 h after starting surgery, and at completion of surgery. Heart rate, MAP, SpO2, PVI, end-tidal pressure of CO2 (PETCO2), urine output, and the type and quantity of fluid supplemented were monitored with the Vigileo monitor at each time point. Arterial blood gas analysis was also carried out at the four time points described above. Serum lactic acid level was monitored by intravenous blood extraction.
Patients were transferred from the ICU when they exhibited a stable cardiovascular and respiratory status, normal acid-base balance, and normal electrolytes. They also had to be ventilator- and vasoactive drug-independent. Once discharged from the ICU, all patients underwent routine fluid management administered by an experienced physician.
All patients received fast-track management. No routine bowel preparation was performed preoperatively. In both groups, clear liquids were encouraged on postoperative day (POD) 1 (beginning 24 h after surgery). On PODs three and four the patients could have a semi-fluid diet and the intravenous feeding was reduced or discontinued. The nasogastric tube was removed on POD 1, and early ambulation was encouraged. Intravenous fluids were administered postoperatively only to the degree that was necessary to avoid dehydration and electrolyte imbalance.
Patients were discharged home when they showed stable cardiovascular and respiratory conditions, they could take oral fluid and food, spontaneous excretion occurred, no infection was present, and their consciousness level was comparable to that of their preoperative state. Two experienced surgeons who were not informed about the patient grouping made these determinations.
Statistical analysis
Generally, continuous data were presented as the mean ± standard deviation (SD) or the median and interquartile range (IQR)—Q1, Q3—if data were not normally distributed. Categoric data were presented as the number (%). Differences between groups were compared using a two-sample t test for continuous data or the Mann–Whitney U test if data were not normally distributed: Pearson’s χ2 test for categoric data or Fisher’s exact test if the cell number was <5. Hemodynamic indexes and arterial blood gas indexes over time were summarized as the mean ± SD and compared between the treatment and control groups using repeated measurement analysis of variance (ANOVA). All statistical assessments were two-tailed, and p < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS version 15.0 software (SPSS, Chicago, IL, USA).
Results
Demographics
Of the 122 patients assessed for eligibility for inclusion in the study, there were ultimately 30 patients each in the GDT and control groups included in the analysis (Fig. 2). The demographic and baseline clinical characteristics of the two groups were similar (Tables 1, 2). There was no significant difference in the preoperative medications between the groups. There was no significant difference between groups regarding the severity of coronary artery disease. Approximately 65–70 % of the patients in each group had one diseased vessel and stenosis severity of 30–70 %. Preoperatively, SpO2 was slightly higher in the GDT group than in the control group: 97.5 (96, 98) vs. 96 (94, 97), p = 0.024. There was no significant difference between groups in regard to the operation time or operation type. No operations in either group were performed laparoscopically.
Adverse cardiac events
A total of 11 patients (36.7 %) in the GDT group had adverse cardiac events, whereas 18 patients (60 %) in the control group had adverse events. The events included serious arrhythmia, congestive heart failure, myocardial ischemia, and acute myocardial infarction. The incidence of adverse cardiac events was not significantly different between groups (all, p > 0.05).
Recovery and discharge
Time to extubation, time to flatulence, time to defecation, time to resumption of liquid diet, length of ICU stay, and length of hospital stay were all shorter in the GDT group than in the control group (all, p < 0.05). In addition, the incidence of severe nausea and vomiting was lwer in the GDT group than in the control group (20 vs. 50 %, p = 0.015) (Table 2).
Fluid administration, hemodynamics, blood gases
Fluid administration by group is summarized in Table 3. Patients in the GDT group received a lower total amount of intraoperative fluid infusion and amount of crystalloids but a higher amount of colloids than the control group (all, p < 0.05). Colloids comprised 38 % of the fluids administered in the GDT group but only 20 % of the fluids administered in the control group. Intraoperative urine output was significantly less in the GDT group (p < 0.001).
Comparisons of hemodynamic indexes and arterial blood gas indexes over time between the GDT and control groups are shown in Tables 4 and 5, respectively. The time trends for MAP, central venous pressure, and stroke volume of the GDT group were significantly different from those of the control group (all p < 0.05) (Table 4). The time trends for the oxygen delivery index (DO2I) and lactic acid were also significantly different in the GDT and control groups (both, p < 0.05) (Table 5).
Discussion
Our hypothesis that GDT would decrease the number of adverse cardiovascular events in elderly patients with coronary artery disease undergoing GI surgery was not substantiated. Although the GDT group experienced fewer adverse cardiac events, the difference between the two groups did not reach statistical significance. However, the lack of significance might have been due to the small number of study patients. Recovery from surgery was faster in the GDP group. Also, GDT was associated with a shorter ICU stay, faster return of GI function, less nausea and vomiting, and better hemodynamic indices than the patients managed with traditional fluid therapy.
The purpose of GDT is to stabilize the hemodynamic status with suitable fluids according to real-time hemodynamic function data. The fluids administered in the GDT group were based on hemodynamic parameters obtained from the Violeo/FloTrac system and the treatment algorithm. The total amount of fluids administered intraoperatively was significantly different between the GDT and control groups (2,650 vs. 3,950 ml, respectively), whereas the amount administered during the first 24 h after surgery was not (2,150 vs. 2,100 ml, respectively). Additionally, the relative amount of crystalloids and colloids used was different between the two groups. The GDT group received a smaller volume of crystalloid and a much higher percentage of colloid than those on conventional therapy. The final amount of colloids may reflect a need for more colloids to stabilize and reduce the edema associated with anastomosis, indicating that the traditional method of fluid management may have flaws. GDT administered via the Violeo/FloTrac system allows more precise administration of fluids than routine fluid therapy, which is especially evident intraoperatively when large fluid shifts are likely to occur and may not be identified by standard monitoring. Postoperatively, less variation in fluid status is likely, and thus the amount of fluid required under the GDT algorithm more closely matches that of routine fluid management.
Patients with prolonged bowel surgery are particularly likely to develop GI edema, which would decrease tissue perfusion and oxygenation [15]. It is possible that edema caused by the greater volume of crystalloids used in the conventional fluid therapy group in this study is at least partly responsible for the slower recovery of bowel function in these patients. Their increased nausea and vomiting is probably due the accompanying slow return of bowel function. Time to discharge from the ICU and from the hospital was relatively long for all patients. This was probably because the patient population was elderly and had coronary heart disease.
Intraoperative hemodynamic perturbations are the main cause of tissue hypoperfusion. They also increase the incidence of postoperative complications and extend the length of hospital stay [1, 2]. Elderly patients with coronary heart disease are highly sensitive to body fluid volume changes during GI surgery. Stress reactions increase myocardial oxygen consumption and the incidence of adverse cardiac events during the perioperative period in patients with coronary heart disease [1]. The vasodilating effect produced by anesthetics and anesthetic methods induces relative hypovolemia. General anesthesia also produces cardiac depression [1, 15]. In addition, preoperative and postoperative fasting and other abnormal fluid losses can result in long-term negative fluid balance [3]. Taken together, these factors may induce severe hemodynamic instability.
Accurate evaluation of fluid status cannot be achieved in patients undergoing GI surgery because of pathophysiologic changes due to the disease itself, preoperative bowel preparation, intraoperative fluid loss, and stress reaction. Normally, the body can compensate for a 25–30 % of loss of effective circulating blood volume [3]. Because the digestive tract is extremely sensitive to changes in fluid volume, loss of 10–15 % of the effective circulating blood volume may induce hypoperfusion of the GI tract [8]. A hypoperfusion of the GI tract cannot be determined through routine monitoring such as HR and BP, GI tract injury during anesthesia may go unrecognized [2].
We used the Violeo/FloTrac monitoring system with selected quantitative indicators such as CI, SVV, SVI, and MAP and carried out GDT based on these data. The Violeo/FloTrac monitoring system uses the arterial pressure cardiac output technique (APCO). Study has shown that there is excellent correlation between APCO and CO measured with the thermodilution method [6]. APCO is based on analysis of the peripheral arterial waveform. The machine continuously monitors and displays CO, CI, SV, SVI, and SVV and automatically adjusts vascular compliance and resistance. Kobayashi et al. [7] reported that the Violeo/FloTrac system has excellent sensitivity and specificity because the SVV is monitored by APCO. When the SVV is 8–12 (normal range), there is excellent correlation between it and fluid expansion, whereas an SVV of >12 indicates inadequate fluid volume [16]. Therefore, an SVV of 8–12 was used as the target value in this study. We also observed the PVI in this study. There was excellent correlation between the PVI and variations in pulse oxygen amplitude, and the PVI could be monitored continuously. The PVI has been shown to be useful for evaluating body fluid volume under general anesthesia with mechanical ventilation and for guiding fluid therapy [17].
Although there are differences between the current study and other related studies in the target value and monitoring means, the results are similar. Pearse et al. [18] monitored CO and DO2I using a pulse-induced contour cardiac output (PiCCO) technique in patients undergoing abdominal surgery. The DO2I was maintained at >600 ml/min/m2 using colloids and crystalloids, and the length of hospital stay and incidence of postoperative complications were significantly lower in the experimental group than in the normal fluid therapy group. Lopes et al. [9] used pulse pressure variation as an indicator of fluid volume and found that when maintained at <10 % the length of hospital stay was significantly reduced in patients undergoing various high risk surgeries. Noblett et al. [10] guided fluid therapy with transesophageal echocardiography and found that it could significantly decrease the length of hospital stay and number of postoperative complications in patients undergoing elective colon resections. Recent meta-analyses have also indicated that GDT can improve outcomes in patients undergoing high-risk surgical procedures [11–13].
GDT is thought to lead to improved outcomes because optimization of hemodynamic parameters results in less tissue hypoxia during the intraoperative and postoperative periods [2, 5, 8]. DO2I is an important physiologic indicator reflecting tissue perfusion and oxygen content. It represents the oxygen delivery rate of the heart to peripheral tissues and reflects the total circulation capacity. We found that DO2I was significantly lower in the control group compared to the GDT group, which suggests that routine fluid therapy leads to deficient intraoperative microcirculation perfusion. However, the control group in our study received more fluid. This unusual finding may be because in traditional fluid management fluid is not supplied in an appropriate volume when it is most required by the body. We also found that the serum lactic acid was significantly lower in the GDT group than in the control group, suggesting that GDT can improve oxygen delivery to tissues. Lactic acid can reflect the balance of tissue oxygen demand and accurately reflects the severity of tissue hypoperfusion and shock. Lactic acid is more sensitive than the HR, arterial pressure, or CO in reflecting hypovolemia. Clinical studies have shown that increased lactic acid levels are associated with mortality and organ failure in critically ill patients [19].
There were some limitations to this study that should be considered. The study was a single-center study with a relatively small sample size. Additionally, the study focused on a specific population: elderly patients with coronary heart disease undergoing GI surgery. Thus, the results may not be generalizable to patients with other medical condition undergoing different surgeries. We excluded certain patient populations (ASA grade >III, age >80, BMI >24 kg/m2, cases in which excessive blood loss was anticipated) because these conditions are associated with increased surgical and anesthetic risk and we did not believe it was ethical to include patients with these attributes to receive what is essentially an experimental method (i.e. GDT). Nitroglycerin use and preoperative dehydration were exclusion criteria: Nitroglycerin influences the Violeo/FloTrac system. Patients who were dehydrated prior to surgery were excluded because we wanted the baseline fluid characteristics of both groups to be similar. Lastly, a power analysis was not performed prior to the study. A post-hoc analysis indicated that the sample size was too small to detect the observed difference in the adverse event rate between the two groups.
Conclusions
In this study of elderly patients with coronary heart disease undergoing GI surgery, GDT was associated with shorter ICU stay, faster return of GI function, less nausea and vomiting, and better hemodynamic indices than in patients managed with traditional fluid therapy. However, GDT was not associated with fewer adverse cardiac events.
References
Kertai MD, Klein J, Bax JJ et al (2005) Predicting perioperative cardiac risk. Prog Cardiovasc Dis 47:240–257
Mythen MG (2005) Postoperative gastrointestinal tract dysfunction. Anesth Analg 100:196–204
Brandstrup B, Svemen C, Engquist A (2006) Hemorrhage and operation cause a contraction of the extracellular space needing replacement: evidence and implications? A systematic review. Surgery 139:419–432
Bundgaard-Nielsen M, Ruhnau B, Secher NH et al (2007) Flow-related techniques for preoperative goal-directed fluid optimization. Br J Anaesth 98:38–44
Mayer J, Boldt J, Mengistu AM et al (2010) Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. J Crit Care 14:R18
Hofer CK, Button D, Weibel L et al (2010) Uncalibrated radial and femoral arterial pressure waveform analysis for continuous cardiac output measurement: an evaluation in cardiac surgery patients. J Cardiothorac Vasc Anesth 24:257–264
Kobayashi M, Koh M, Irinoda T et al (2009) Stroke volume variation as a predictor of intravascular volume depression and possible hypotension during the early postoperative period after esophagectomy. Ann Surg Oncol 16:1371–1377
Wakeling HG, McFall MR, Jenkins CS et al (2005) Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 95:634–642
Lopes MR, Oliveira MA, Pereira VO et al (2007) Goal directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care 11:R100
Noblett SE, Snowden CP, Shenton BK et al (2006) Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg 93:1069–1076
Corcoran T, Rhodes JE, Clarke S et al (2012) Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg 114:640–651
Challand C, Struthers R, Sneyd JR et al (2012) Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth 108:53–62
Giglio MT, Marucci M, Testini M et al (2009) Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth 103:637–646
Lees N, Hamilton M, Rhodes A (2009) Clinical review: goal-directed therapy in high risk surgical patients. Crit Care 13:231
Kaye AD, Riopelle JM et al (2009) Intravascular fluid and electrolyte physiology. In: Miller RD, Erikson LI, Fleisher LA (eds) Miller’s anesthesia, vol 7. Elsevier Churchill Livingstone, Philadelphia, pp 1705–1737
Chakravarthy M, Patil TA, Jayaprakash K et al (2007) Comparison of simultaneous estimation of cardiac output by four techniques in patients undergoing off-pump coronary artery bypass surgery: a prospective observational study. Ann Card Anaesth 10:121–126
Cannesson M, Desebbe O, Rosamel P et al (2008) Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth 101:200–206
Pearse R, Dawson D, Fawcett J et al (2005) Early goal-directed therapy after major surgery reduces complications and hospital stay: a randomized, controlled trial. Crit Care 9:R687–R693
Soliman HM, Vincent JL (2010) Prognostic value of admission serum lactate concentrations in intensive care unit patients. Acta Clin Belg 65:176–181
Acknowledgments
This study was supported by the Research Fund for the Doctoral Program of Higher Education (no. 20126517110005).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, H., Guo, H., Ye, Jr. et al. Goal-Directed Fluid Therapy in Gastrointestinal Surgery in Older Coronary Heart Disease Patients: Randomized Trial. World J Surg 37, 2820–2829 (2013). https://doi.org/10.1007/s00268-013-2203-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2203-6