Abstract
Background
Elevated lactate and interleukin-6 (IL-6) levels were shown to correlate with mortality and multiple organ dysfunction in severely traumatized patients. The purpose of this study was to test whether an association exists between 24-hour lactate clearance, IL-6 and procalcitonin (PCT) levels, and the development of infectious complications in trauma patients.
Methods
A total of 1757 consecutive trauma patients with an Injury Severity Score (ISS) > 16 admitted over a 10-year period were retrospectively analyzed over a 21-day period. Exclusion criteria included death within 72 h of admission (24.5%), late admission > 12 h after injury (16%), and age < 16 years (0.5%). Data are stated as the median (range).
Results
Altogether, 1032 trauma patients (76.2% male) with an average age of 38 years, a median ISS of 29 (16–75), and an Acute Physiology, Age, and Chronic Health Evaluation (APACHE) II score of 14 (0–40) were evaluated. The in-hospital mortality (>3 days) was 10%. Patients with insufficient 24-hour lactate clearance had a high rate of overall mortality and infections. Elevated early serum procalcitonin on days 1 to 5 after trauma was strongly associated with the subsequent development of sepsis (p < 0.01) but not with nonseptic infections. The kinetics of IL-6 were similar to those of PCT but did differentiate between infected and noninfected patients after day 5.
Conclusions
This study demonstrates that elevated early procalcitonin and IL-6 levels and inadequate 24-hour lactate clearance help identify trauma patients who develop septic and nonseptic infectious complications. Definition of specific cutoff values and early monitoring of these parameters may help direct early surgical and antibiotic therapy and reduce infectious mortality.
Similar content being viewed by others
Introduction
Early recognition of trauma patients at risk for the development of infectious complications remains a difficult task. Diagnosis of infection and sepsis can be troublesome in severely traumatized patients: Positive bacteriologic specimens may be late or absent, the clinical interpretation of local colonization may be ambiguous, and traditional infection markers such as core body temperature and white blood cell (WBC) counts may show nonspecific alterations. Classic laboratory parameters, such as admission lactate level, base deficit, and pH, have been extensively investigated with respect to their prognostic significance for various endpoints, such as sepsis, organ dysfunction, or death [1–5]. In severely injured patients with an Injury Severity Score (ISS) >16, serial measurements of one or a combination of such parameters are helpful for identifying patients at risk of occult hypoperfusion, subsequent organ dysfunction, and death. Early on, a correlation was reported between serum lactic acidosis, the development of septic shock, and subsequent death in trauma patients [6–8].
Persistent occult hypoperfusion (POH) in trauma patients has been proposed to induce a state of relative immunosuppression, leading to subsequent infection. POH has been associated with an increase in infections in trauma patients in a study by Claridge et al. [9], and the authors demonstrated a good correlation between the time necessary for correction of lactic acidosis and the appearance of subsequent infection.
With respect to the differentiation between systemic inflammatory response syndrome (SIRS) and sepsis, procalcitonin (PCT) has been a focus of interest in recent years. PCT is a precursor of calcitonin, a hormone produced by the C-cells of the thyroid gland that is essential for calcium metabolism [10]. The secretion of PCT is not limited to the thyroid gland and does not occur in healthy individuals without significant bacteremia [11]. The blood concentrations of PCT were also reported to show a good relation with the degree of sepsis [12]. In the acutely injured patient, PCT was shown to be helpful for differentiating between SIRS and sepsis, and it has outperformed parameters such as interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNFα) in terms of sensitivity (85%) and specificity (91%), as reported by Balci et al. [13].
Interleukin-6, our second parameter of interest, is a proinflammatory cytokine produced by T cells and macrophages to stimulate immune responses to tissue damage [14]. Certain microbial molecules, termed pathogen-associated molecular patterns (PAMPs), bind to Toll-like receptors on various immune cells, thereby inducing intracellular signaling cascades, which trigger the production of inflammatory cytokines. IL-6 then exerts its various proinflammatory effects as part of the acute-phase response through binding to its membrane-bound or soluble IL-6 receptor. Among the best studied effects of IL-6 is the mobilization of energy precursors from muscle and fat tissues, leading to increased core body temperature [15]. Both SIRS and sepsis can lead to elevated serum IL-6 levels, with peak values recorded in patients with septic shock [16]. IL-6 has further been shown to be an independent predictor of outcome in unselected critically ill patients [17]; but despite its obvious diagnostic advantages in the intensive care unit (ICU) setting, it has yet to become a parameter of everyday use in clinical practice.
The aim of this study was to assess whether PCT and IL-6 have a relation with infectious and/or septic complications in our trauma patient collective. Furthermore, we hypothesized that impaired 24-hour lactate clearance may be associated with an increased rate of infectious complications in trauma patients. Elevated admission lactate level measurements have been shown to correlate with fatal outcome and organ failure in trauma patients [4]. In addition, serial measurements of lactate or lactate clearance over time may provide a better indicator of organ failure and mortality in critically ill patients [18–21]. The ideal threshold by which to define adequate lactate clearance in critically injured patients, however, remains unresolved.
Patients and methods
We retrospectively analyzed the charts and serum specimens of 1757 severe trauma patients admitted to the University of Zürich Hospital trauma service between January 1, 1996 and December 31, 2005. All trauma patients aged 16 years and older with an ISS > 16, a threshold commonly used to define severely injured patients, were routinely entered into the University of Zürich Hospital trauma database. Prior to data acquisition, the study was approved by the ethics committee of the University of Zürich.
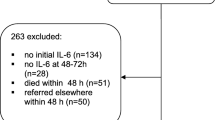
Exclusion criteria included burn injuries, death within 72 h of admission (24.5%), admission after more than 12 h following trauma (16% of the initial patient collective), and age < 16 years. Finally, 1032 patients were included. We registered and analyzed vital parameters, daily routine, and laboratory parameters as well as trauma and organ dysfunction scores and indices as outlined in Table 1. Outcome parameters included length of hospital stay, length of ICU stay and duration of mechanical ventilation, overall mortality rates, cause of death, and morbidity grouped into specific infectious and/or organ dysfunction-related complications.
Initial stabilization and treatment was performed according to Advanced Trauma Life Support (ATLS) guidelines. Basic radiography studies, as well as abdominal ultrasonography were performed; computed tomography (CT) scans were obtained for complex injuries in hemodynamically stable patients. If hemodynamic stabilization of the patient failed, immediate surgical therapy according to damage control principles followed under supervision of the attending trauma surgeon. Following initial surgical therapy, patients were transferred to a specialized trauma ICU for further stabilization and monitoring.
Definition of infectious complications
Criteria for infection varied depending on the site of infection. Pneumonia was diagnosed when a predominant organism was isolated from appropriately obtained sputum cultures in the setting of purulent sputum production and/or a new or changing pulmonary infiltrate on chest radiography. Bloodstream infections were diagnosed when the predominant organism was identified in blood cultures obtained under sterile conditions. Criteria for urinary tract infections (UTIs) included isolation of >105 organisms/ml urine or >104 organisms in patients with symptoms typical for UTIs. Criteria for catheter-related infection included isolation of >5 colony forming units (CFU) from catheter tips cultured only in the setting of suspected infection. SIRS and sepsis were diagnosed as previously defined in the literature [22]. Sepsis was diagnosed in patients with an established (culture-positive) localized or systemic infection together with four positive SIRS criteria during three consecutive days. This strict definition of SIRS was chosen to exclude patients exhibiting a short state of systemic inflammation as commonly observed following severe trauma or major surgery [23].
Laboratory measurements
Blood gas analysis and lactate measurements were obtained using standard radiometers (ABL 800 Flex; Drott Medizintechnik, Wien Neudorf, Austria). PCT, IL-6, and CRP were measured using commercially available kits according to the manufacturer’s instructions: PCT: LUMItest PCT, Brahms Diagnostica, Berlin Germany; IL-6: IL-6 DuoSet ELISA, R + D Systems, Minneapolis, MN, USA). Routine hematologic and blood chemistry analyses (differential WBC count, CRP) were performed by the clinical hematology and chemistry laboratories at the University Hospital of Zürich.
24-Hour lactate clearance
Arterial lactate levels were measured consecutively from day 0 to day 21. A threshold of 2.5 mmol/l was used as a cutoff value by which to define lactate clearance within 24 h following admission, as previously suggested by others [24, 25]. According to the individual admission and 24-hour lactate values, patients were divided into four groups as shown in Fig. 2b: (1) always below 2.5 mmol/l; (2) decreasing to below 2.5 mmol/l (starting above 2.5 mmol/l but decreasing to below 2.5 mmol/l within 24 h); (3) increasing to above 2.5 mmol/l (starting below 2.5 mmol/l but increasing to above 2.5 mmol/l within 24 h), and (4) always above 2.5 mmol/l. Outcome parameters in this analysis were infection or sepsis as defined above, and death. A second analysis of our lactate clearance data focused on its potential association with the length of hospital and ICU stays and the length of mechanical ventilation. Again, a threshold of 2.5 mmol/l within 24 h was used as a cutoff value by which to define lactate clearance.
Comparison of commonly used laboratory markers of inflammation and infection in trauma patients. *Significant difference (p < 0.01), no-infection group vs. sepsis group. † p < 0.01, infection group vs. sepsis group; ‡ p < 0.05, no-infection group vs. sepsis group. § p < 0.05, infection group vs. sepsis group. II p < 0.05, no-infection group vs. infection group. a Serum procalcitonin levels. b Serum interleukin-6 levels. c Leukocyte count. d Serum C-reactive protein values
Statistical analysis
Serum parameters were logarithmically transformed before analysis. One-way analysis of variance (ANOVA) then was used to detect differences at various time points, followed by the Holm-Sidak test to isolate the group or groups that differ from the others. A value of p < 0.05 was regarded as statistically significant. To address the problem of multiple comparisons at different days, p < 0.01 was considered statistically significant.
Pearson’s chi-squared test followed by Fisher’s exact test with Bonferroni correction was used to identify significant changes between two corresponding groups. For data that were not normally distributed, the Kruskal-Wallis test or the Mann-Whitney rank-sum test (according to the number of groups) was applied. Stepwise logistic regression was then performed using the following parameters: age, sex, ISS, mean arterial pressure (MAP), systolic blood pressure, Glasgow Coma Scale (GCS), PCT, IL-6, and lactate clearance group always >2.5 mmol/l. For the stepwise logistic regression, IL-6 and PCT values from days 1 and 2 as well as values from days 2 and 3 were combined. Receiver operating characteristic (ROC) curves were generated for the parameters listed above for days 1 and 2. Data are presented as the median (range) or mean ± standard error of the mean (SEM), as appropriate. Statistical analysis was performed using both SigmaStat 3.01.0 (Systat Software, Richmond, CA, USA) and SPSS 15.0 software (SPSS, Chicago, IL, USA).
Results
An overview of the patient collective enrolled into this study is provided in Table 1. Most of the patients were male (76.2%) and suffered from blunt trauma (90.4%); 10.2% died from their injuries. Head and neck trauma accounted for most of the fatal injuries.
Comparison of IL-6 and PCT with common clinical immunologic parameters
Procalcitonin levels are depicted in Fig. 1a. The highest PCT values were observed on day 1 in patients who later developed septic infections (i.e., 3.82 ± 0.99 ng/ml in the sepsis group versus 0.81 ± 0.1 ng/ml in controls without infection on day 1, p < 0.0001). PCT values in subsequently septic patients were also higher than PCT levels recorded in patients with isolated infections up until day 5 after trauma. There is no significant difference between PCT values in patients with nonseptic infections (1.54 ± 0.55 ng/ml) and in patients without infection (p = 0.97) on day 1 or on any following day. PCT values decreased in all groups after day 1 but were still significantly different between the sepsis group and the two other groups until day 5 after trauma (p < 0.01 for every day).
a Arterial lactate measurements as a function of the time following trauma. Day 0 lactate is defined as the first measured lactate upon arrival in the intensive care unit (ICU), which is usually lower than the initial value measured in the trauma room (see Table 1, admission value). *p < 0.01, no-infection group vs. sepsis group. †p < 0.01, infection group vs, sepsis group. ‡p < 0.05, no-infection group vs. sepsis group. §p < 0.05, infection group vs. sepsis group. IIp < 0.05 no-infection group vs. infection group. b Development of lactate levels within the first 24 h following admission. Grouping was performed according to the development of arterial lactate levels within 24 h following admission (e.g., “increasing above 2.5 mmol/l” = initial lactate < 2.5 mmol/l, 24-hour lactate > 2.5 mmol/l). *p < 0.013, “always below” group vs. “always above” group. c Association between 24-hour lactate clearance and length of hospital stay, ICU stay, and length of mechanical ventilation. *p < 0.05, “always below 2.5 mmol/l” group vs. “decreasing below 2.5 mmol/l” group. †p < 0.0001, “always below 2.5 mmol/l” group vs. “always above 2.5 mmol/l” group
With respect to IL-6 (Fig. 1b), patients with a septic course showed highest levels on day 1 after trauma (551.6 ± 124.1 ng/l compared to 282.1 ± 39.8 ng/l in patients without infections, p = 0.28). However, the difference between subsequently septic patients and noninfected patients reached statistical significance only between days 3 and 7, after which all three groups slowly returned to baseline values.
Figure 1c illustrates WBC counts. During the initial 5 days, a significant decrease in the WBC count was observed in all three groups compared to admission (day 0) values. The lowest values were reached on day 1 in the sepsis group (8.01 ± 0.28 × 103 cells/μl) compared to the no-infection group (9.75 ± 0.17 × 103 cells/μl; p < 0.0001) and the nonseptic infection group (8.63 ± 0.26 × 103 cells/μl; p = 0.008). From day 7 onward, patients in the sepsis group displayed higher WBC counts than the other two patient groups, although these differences approached statistical significance only on days 7 and 14 (both p < 0.05).
C-reactive protein levels (Fig. 1d) showed a steady increase in all three groups following trauma, reaching peak levels around 150 mg/dl between days 3 and 7. On day 0, we found slightly (but approaching significantly) lower CRP levels in the sepsis group (7.3 ± 2.52 mg/dl) compared to the no-infection group (9.58 ± 1.25 mg/dl; p = 0.022). After day 7, CRP levels returned to baseline levels earlier in noninfected patients than in those with localized infections or sepsis, whose levels were significantly higher on days 5, 7, and 14 (all p < 0.01). Even on day 21, CRP levels averaged 90 mg/dl in the sepsis group versus 62 mg/dl in the no-infection group, indicating the long period of recovery from both sepsis and major trauma in general.
Blood lactate and 24-hour lactate clearance
Figure 2a shows the course of lactate concentrations over 14 days following major trauma, with the highest levels upon admission in all groups. Admission lactate levels in subsequently septic patients (2.27 ± 0.12 mmol/l) were significantly higher than those observed in patients without infections (1.89 ± 0.07 mmol/l; p = 0.006) and remained highest in this group until day 5 after trauma (all p < 0.01). Patients who developed localized infections trended to higher lactate levels on days 1 and 3 after trauma when compared to patients who did not develop any infections (p = 0.011 and 0.022, respectively, for days 1 and 3). However, all groups displayed “physiologic” lactate values (<1.2 mmol/l) after day 3, and any observed differences from this day onward were minor in magnitude and potentially without clinical significance.
Figure 2b illustrates 24-hour lactate clearance using a cutoff value of 2.5 mmol/l. The rate of infectious complications in all groups was at least at 39%, reaching its highest value in the “always above 2.5 mmol/l” group with an infection rate of 70% (p < 0.0001). The mortality rate in the “increasing above 2.5 mmol/l” group was twice as high (21%) as the mortality rate in the other three groups (around 10%), but this difference did not reach statistical significance. The rate of septic infections, on the other side, was markedly lower in the “always below 2.5 mmol/l group” (19.3%) than in the “always above 2.5 mmol/l group (53.7%, p < 0.0001).
Figure 2c shows the relation between 24-hour lactate clearance and the length of hospital and ICU stays and the length of mechanical ventilation. We found that patients with continuously high (always above 2.5 mmol/l) lactate levels stayed in the hospital the longest (38.4 ± 3.2 days), which was significant especially when compared to patients with always low lactate levels (24.1 ± 0.8 days, p < 0.0001). The longest ICU stay and longest need for mechanical ventilation were seen in the group of patients with lactate levels always above 2.5 mmol/l (27.8 ± 6 and 23.0 ± 5 days, respectively; p < 0.0001 for both parameters compared to the group of patients with lactate levels always below 2.5 mmol/l).
Logistic regression and ROC curve
Stepwise logistic regression from days 1 + 2 support the results from the initial univariate analysis; ISS (p = 0.013), IL-6 (p = 0.008), and PCT (p = 0.032) remained independent predictors of septic infections in our patient population. On days 2 + 3, both IL-6 (p < 0.001) and lactate clearance “always above 2.5 mmol/l” (p = 0.013) were independent predictors of sepsis. The ROC curve is shown in Fig. 3, with the bold line marking the prognostic index of the assessed parameters. The comparably low sensitivity of lactate clearance “always above 2.5 mmol/l” reflects the low patient numbers in this subgroup (n = 13).
Discussion
The definition of specific parameters by which to identify trauma patients at risk of developing infectious complications and sepsis at an early stage remains a crucial goal of laboratory research in trauma surgery. We have previously shown that PCT is a sensitive and predictive indicator of sepsis and severe MODS in trauma patients [26]. Its use in the early distinction between SIRS and sepsis has further facilitated the identification of patients requiring antibiotic therapy or surgical intervention to control infectious foci [13]. The data from our present study show that trauma patients who later develop septic complications have initially high PCT levels and fail to clear initially elevated blood lactate levels within 24 h. PCT shows peak levels within the first 48 h following trauma, whereas WBC counts and CRP levels increase later and fail to distinguish between septic and nonseptic patients during the first days.
Interleukin-6 may help identify patients at risk for septic complications after at least 3 days following trauma, but its association was, in our series of patients, less stringent when compared to PCT as an isolated parameter. Logistic stepwise regression confirmed this association; PCT in the early days after severe trauma is an independent predictor of septic infections, making it a useful prognostic parameter during the early treatment of such patients. IL-6 as another independent predictor of sepsis during the first 3 days after major trauma deserves similar attention despite its so far uncommon use in everyday clinical practice.
The ISS is another predictor of sepsis in our patient cohort, as had been shown previously [27].
Herein may lie the advantage of the combined use of PCT and IL-6; together, these parameters help differentiate patients with a subsequently septic course from patients who develop localized, nonseptic infections. These findings are strikingly similar to a series of 90 trauma patients reported by Meisner et al. [28], who found characteristics of PCT and CRP levels comparable to those recorded in our patients. Those authors showed a significant association between early increases in PCT and sepsis, especially septic shock. CRP levels failed to indicate specific infectious complications in their trial, although overall both PCT and CRP levels tended to be higher in nonsurvivors than survivors [28]. Castelli et al., in their comparison of the diagnostic value of PCT and CRP in 150 ICU patients, came to similar conclusions but found that CRP concentrations were induced nearly to their maximum during less severe symptoms of systemic inflammation and organ dysfunction and did not further increase during more severe stages of the disease [29].
The use of PCT as a diagnostic tool to differentiate between SIRS and sepsis has been questioned by several authors [30, 31]. Suprin et al. found sensitivity and specificity values as low as 65% and 70% for PCT using 2.0 ng/ml as a cutoff value, with similar results for the use of CRP [30]. Tang et al. reported 71% sensitivity and specificity for PCT in their meta-analysis of 18 clinical trials [31]. The authors found an “upwardly biased” performance of PCT in smaller studies, which moved toward a null effect in larger trials; the authors concluded that the present data may not support the current widespread use of PCT in ICU practice.
Other authors came to more favorable conclusions regarding the specific use of PCT in selected patient populations [32, 33]. Svoboda et al. showed a significant decrease in ICU mortality in 72 patients with a suspected septic focus following major injury or multiple surgery by using PCT as an aid to deciding on early reintervention [32]. The control group in this trial underwent the same clinical and laboratory reevaluation but without PCT measurement. The group receiving PCT-guided treatment further benefited from a shorter ICU stay and fewer days on the ventilator, although these differences were not significant. A recent meta-analysis by Uzzan et al., however, concluded from 49 clinical trials that PCT indeed aids the clinical diagnosis of sepsis and septic shock in postoperative and trauma patients [33]. The authors underlined the need to incorporate biomarkers such as PCT into an overall assessment of critically ill patients rather than in preference to clinical evaluation. In this study we showed that PCT is capable of identifying patients at risk of infectious, especially septic, complications at the beginning of their recovery period, which may further aid in the identification of patients at risk of such complications.
Interleukin-6 has been extensively investigated as a marker of systemic inflammation following trauma and surgery. Its kinetics during the early days following major trauma are well documented [14, 34–36], and several studies have shown a significant correlation between peak IL-6 levels and the development of SIRS and sepsis in such patients [16, 36, 37]. Animal studies have shown that concentrations of IL-6 correlate with the extent of injury [38], and the authors of a recent study have even proposed the use of IL-6 as a forensic tool to distinguish between traumatic and non-trauma-related deaths [39].
In our data collection, we found comparable results with the highest IL-6 levels on day 1 in patients who later developed sepsis. From a clinical point of view, however, it is important to note that, in contrast to PCT, IL-6 does not indicate the presence of bacterial infection and may not be (mis-)used in trying to diagnose occult infections [33]. The value of IL-6 is as an index of systemic inflammation, which in itself has prognostic significance. A recent comparison of the prognostic value of IL-6, PCT, and CRP in febrile ICU patients demonstrated that IL-6 was a better predictor of mortality in this group of patients, and the authors suggested its use as a tool to identify patients who could benefit from intensive monitoring and early specific therapy [40].
The third focus of our study was on the use of arterial lactate, its clearance within 24 h, and its association with infectious complications in our patient collective. We found that initial lactate levels can differentiate between patients with an eventually septic course and those without it. Moreover, its course over the first 24 h provided a tool to identify patients at risk for the development of infection, sepsis, and death. Similar to the incidence of specific infectious complications, we found that failed or insufficient lactate clearance (<2.5 mmol/l) within 24 h is associated with significantly longer hospital and ICU stays and increased duration of mechanical ventilation. These findings are further supported by the fact that failed 24-hour lactate clearance is another predictor of septic infections in our patient cohort. The threshold of 2.5 mmol/l at 24 h following injury was chosen based on a series of preliminary analyses using different concentrations of lactate at time points between 6 h and up to 48 h. The most consistent results were found after 24 h using 2.5 mmol/l, which is consistent with findings from two other trials, in which this threshold was used [24, 25]. Kobayashi et al. studied 22 patients with SIRS and focused on the development of disseminated intravascular coagulation (DIC) and death as a function of serial lactate measurements [24]. The authors found that a lactate concentration of 2.5 mmol/l on day 1 provided an optimal cutoff point, defined as the best prognostic value for predicting subsequent death in patients with SIRS.
In line with our findings, Durham et al. showed in 2003 that elevated 24-hour lactate levels can predict better the incidence of both single and multiple organ failure in trauma patients than admission lactate levels alone in their study of 869 patients treated at a Level I trauma center [21]. Using multivariate regression analysis, the authors found that only the Acute Physiology, Age, and Chronic Health Evaluation (APACHE) III, total blood products transfused within 24 h, and lactate levels at 24 h were associated with multiple organ failure. However and unfortunately, a more in depth analysis of lactate clearance at different thresholds is missing in their analysis. With respect to the diagnostic value of admission lactate values, a thorough analysis was conducted by Pal et al. recently [1]. In their study on 6000 trauma patients, the authors failed to show useful predictive values for hospital death using admission arterial lactate as a single parameter after stratification for age, sex, and ISS, among other parameters [1]. Similar to our results, high lactate values were also found in patients who died, but considerable overlap existed, preventing raw lactate values from accurately discriminating between survivors and nonsurvivors. A point to mention is that this study included all trauma admissions regardless of the ISS, thereby comprising a large number of minimally injured patients, and the authors did indeed recommend using lactate levels as a parameter of occult hypoperfusion in ICU trauma patients.
Finally, Phua et al. have recently demonstrated that a concurrent increase of both PCT and lactate between days 1 and 2 after trauma had the highest value in predicting 28-day mortality in patients with septic shock [41]. The combination of the two parameters superseded all independent cytokine measurements or clinical severity scores in their trial of 72 patients. Similar to our findings, the authors found only poor prognostic utility of baseline lactate values. Unfortunately, although serial lactate measurements over the first 72 h were recorded, no specific analysis of lactate clearance was performed in their trial.
The limitations of our study should be noted. First, patient data were collected during a 10-year period, during which the care of trauma patients has continuously evolved. Despite such changes in the clinical management, overall mortality in our trauma patient collective has not significantly changed during this time period. In addition, both the head of our Division of Trauma Surgery and that of Surgical Intensive Care did not change during this period, providing consistency with respect to the clinical management of trauma patients in our institution. Second, patients with severe trauma comprise a highly heterogeneous group of critically ill patients who may exhibit different immunologic responses depending on the injury sustained. We tried to control for this fact by selecting only adult trauma patients who survived at least 72 h and not including late referrals from other hospitals, pediatric patients, or those with burn injuries, thereby excluding the more obviously differing subgroups.
Conclusions
We found a significant positive association between early elevations of both PCT and IL-6 and infectious complications in our collective of 1032 trauma patients. Similarly, delayed or absent 24-hour lactate clearance helped identify patients at risk of infectious complications and of subsequently prolonged ICU and hospital stays. Despite the ongoing strong interest for PCT as a reliable early marker of bacterial infection in critically ill patients, we believe that a combination of clinical and select laboratory parameters yields better prognostic value and provides a tool by which to guide specific therapeutic interventions.
References
Pal JD, Victorino GP, Twomey P et al (2006) Admission serum lactate levels do not predict mortality in the acutely injured patient. J Trauma 60:583–587
FitzSullivan E, Salim A, Demetriades D et al (2005) Serum bicarbonate may replace the arterial base deficit in the trauma intensive care unit. Am J Surg 190:941–946
Martin MJ, FitzSullivan E, Salim A et al (2006) Discordance between lactate and base deficit in the surgical intensive care unit: which one do you trust? Am J Surg 191:625–630
Aslar AK, Kuzu MA, Elhan AH et al (2004) Admission lactate level and the APACHE II score are the most useful predictors of prognosis following torso trauma. Injury 35:746–752
Kaplan LJ, Kellum JA (2004) Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med 32:1120–1124
Manikis P, Jankowski S, Zhang H et al (1995) Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am J Emerg Med 13:619–622
Oppenheim WL, Williamson DH, Smith R (1980) Early biochemical changes and severity of injury in man. J Trauma 20:135–140
Stoner HB, Frayn KN, Barton RN et al (1979) The relationships between plasma substrates and hormones and the severity of injury in 277 recently injured patients. Clin Sci (Lond) 56:563–573
Claridge JA, Crabtree TD, Pelletier SJ et al (2000) Persistent occult hypoperfusion is associated with a significant increase in infection rate and mortality in major trauma patients. J Trauma 48:8–14
Maruna P, Nedelnikova K, Gurlich R (2000) Physiology and genetics of procalcitonin. Physiol Res 49(Suppl 1):S57–S61
Morgenthaler NG, Struck J, Chancerelle Y et al (2003) Production of procalcitonin (PCT) in non-thyroidal tissue after LPS injection. Horm Metab Res 35:290–295
Meisner M, Tschaikowsky K, Palmaers T et al (1999) Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care 3:45–50
BalcI C, Sungurtekin H, Gurses E et al (2003) Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit Care 7:85–90
Ertel W, Faist E, Nestle C et al (1990) Kinetics of interleukin-2 and interleukin-6 synthesis following major mechanical trauma. J Surg Res 48:622–628
Lyngso D, Simonsen L, Bulow J (2002) Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol 543:379–386
Oda S, Hirasawa H, Shiga H et al (2005) Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine 29:169–175
Dimopoulou I, Orfanos S, Kotanidou A et al (2008) Plasma pro- and anti-inflammatory cytokine levels and outcome prediction in unselected critically ill patients. Cytokine 41:263–267
Fink MP (1996) Does tissue acidosis in sepsis indicate tissue hypoperfusion? Intensive Care Med 22:1144–1146
Gore DC, Jahoor F, Hibbert JM et al (1996) Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability. Ann Surg 224:97–102
Gutierrez G, Wulf ME (1996) Lactic acidosis in sepsis: a commentary. Intensive Care Med 22:6–16
Durham RM, Moran JJ, Mazuski JE et al (2003) Multiple organ failure in trauma patients. J Trauma 55:608–616
Collignon PJ, Munro R (1989) Laboratory diagnosis of intravascular catheter associated sepsis. Eur J Clin Microbiol Infect Dis 8:807–814
Lenz A, Franklin GA, Cheadle WG (2007) Systemic inflammation after trauma. Injury 38:1336–1345
Kobayashi S, Gando S, Morimoto Y et al (2001) Serial measurement of arterial lactate concentrations as a prognostic indicator in relation to the incidence of disseminated intravascular coagulation in patients with systemic inflammatory response syndrome. Surg Today 31:853–859
Shapiro NI, Howell MD, Talmor D et al (2005) Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med 45:524–528
Wanner GA, Keel M, Steckholzer U et al (2000) Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit Care Med 28:950–957
Ertel W, Keel M, Marty D et al (1998) Significance of systemic inflammation in 1,278 trauma patients. Unfallchirurg 101:520–526
Meisner M, Adina H, Schmidt J (2006) Correlation of procalcitonin and C-reactive protein to inflammation, complications, and outcome during the intensive care unit course of multiple-trauma patients. Crit Care 10:R1
Castelli GP, Pognani C, Meisner M et al (2004) Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care 8:R234–R242
Suprin E, Camus C, Gacouin A et al (2000) Procalcitonin: a valuable indicator of infection in a medical ICU? Intensive Care Med 26:1232–1238
Tang BM, Eslick GD, Craig JC et al (2007) Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 7:210–217
Svoboda P, Kantorova I, Scheer P et al (2007) Can procalcitonin help us in timing of re-intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology 54:359–363
Uzzan B, Cohen R, Nicolas P et al (2006) Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med 34:1996–2003
Ayala A, Wang P, Ba ZF et al (1991) Differential alterations in plasma IL-6 and TNF levels after trauma and hemorrhage. Am J Physiol 260:R167–R171
Baigrie RJ, Lamont PM, Kwiatkowski D et al (1992) Systemic cytokine response after major surgery. Br J Surg 79:757–760
Schluter B, Konig B, Bergmann U et al (1991) Interleukin 6-a potential mediator of lethal sepsis after major thermal trauma: evidence for increased IL-6 production by peripheral blood mononuclear cells. J Trauma 31:1663–1670
Damas P, Ledoux D, Nys M et al (1992) Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg 215:356–362
Pasquale MD, Cipolle MD, Monaco J et al (1996) Early inflammatory response correlates with the severity of injury. Crit Care Med 24:1238–1242
Mimasaka S, Funayama M, Hashiyada M et al (2007) Significance of levels of IL-6 and IL-8 after trauma: a study of 11 cytokines post-mortem using multiplex immunoassay. Injury 38:1047–1051
Fraunberger P, Wang Y, Holler E et al (2006) Prognostic value of interleukin 6, procalcitonin, and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock 26:10–12
Phua J, Koay ES, Lee KH (2008) Lactate, procalcitonin, and amino-terminal pro-B-type natriuretic peptide versus cytokine measurements and clinical severity scores for prognostication in septic shock. Shock 29:328–333
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Billeter, A., Turina, M., Seifert, B. et al. Early Serum Procalcitonin, Interleukin-6, and 24-Hour Lactate Clearance: Useful Indicators of Septic Infections in Severely Traumatized Patients. World J Surg 33, 558–566 (2009). https://doi.org/10.1007/s00268-008-9896-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-008-9896-y