Abstract
Background
Little evidence is available regarding the relations among circulating monocytes, dendritic cells (DCs), and bacterial translocation (BT) in patients with intestinal obstruction.
Methods
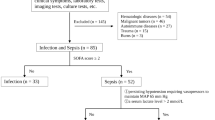
We investigated alterations in DCs in mesenteric lymph nodes (MLNs), circulating immune cells (white blood cell, lymphocyte, and monocyte counts), and BT to MLNs in 21 patients undergoing abdominal surgery because of intestinal obstruction. We also examined whether BT correlated with the development of perioperative systemic inflammatory response syndrome (SIRS) and postoperative septic complications.
Results
BT subsequent to intestinal obstruction was observed in 7 (33%) patients. Preoperative circulating immune cell counts were significantly lower in BT-positive patients than those in BT-negative patients. The presence of preoperative SIRS was also significantly related to BT-positive status. A preoperative monocyte count <290/mm3 was the best predictive factor for BT in MLNs during intestinal obstruction: sensitivity 85.7%; specificity 92.3%; positive and negative predictive values 85.7% and 92.9%, respectively. The area under the receiver operating characteristic curve was 0.944. The expression of S-100 protein-positive DCs in MLNs significantly increased in BT-positive patients.
Conclusions
A significant inverse correlation was observed between the circulating monocyte count and the ratio of DCs among all cells in MLNs (r 2= 0.259). Postoperative septic complications were 3.3 times more common in BT-positive patients than in BT-negative patients. A significant increase in the expression of DCs in MLNs was observed in patients with BT subsequent to intestinal obstruction. Our findings suggested that a low monocyte count (<290 /mm3) and the presence of preoperative SIRS might be useful factors for predicting BT in patients with intestinal obstruction.




Similar content being viewed by others
References
Deitch EA, Bridges WM, Ma JW, et al. (1990) Obstructed intestine as a reservoir for systemic infection. Am J Surg 159:394–401
Horgan AF, Stuart RC, O’Shaughnessy EM, et al. (1994) Bacterial translocation during peroperative colonic lavage of the obstructed rat colon. Br J Surg 81:1796–1798
Deitch EA (1989) Simple intestinal obstruction causes bacterial translocation in man. Arch Surg 124:699–701
Sagar PM, MacFie J, Sedman P, et al. (1995) Intestinal obstruction promotes gut translocation of bacteria. Dis Colon Rectum 38:640–644
Swank GM, Deitch EA (1996) Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg 20:411–417
MacFie J, Reddy BS, Gatt M, et al. (2006) Bacterial translocation studied in 927 patients over 13 years. Br J Surg 93:87–93
Kirkland KB, Briggs JP, Trivette SL, et al. (1999) The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 20:725–730
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Shamoto M, Shinzato M, Hosokawa S, et al. (1992) Langerhans cells in the lymph node: mirror section and immunoelectron microscopic studies. Virchows Arch B Cell Pathol Mol Pathol 61:337–341
Lu JB, Shi HP (2002) Dendritic cells: another possible carrier for gastrointestinal bacterial translocation. Di Yi Jun Yi Da Xue Xue Bao 22:17–19
Sallusto F, Cella M, Danieli C, et al. (1995) Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182:389–400
Winzler C, Rovere P, Rescigno M, et al. (1997) Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med 185:317–328
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:109–1118
Leon B, Lopez-Bravo M, Ardavin C (2005) Monocyte-derived dendritic cells. Semin Immunol 17:313–318
Takesue Y, Kakehashi M, Ohge H, et al. (2005) Bacterial translocation: not a clinically relevant phenomenon in colorectal cancer. World J Surg 29:198–202
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Shimizu T, Endo Y, Tabata T, et al. (2005) Diagnostic and predictive value of the silkworm larvae plasma test for postoperative infection following gastrointestinal surgery. Crit Care Med 33:1288–1295
Nakayama Y, Inoue Y, Minagawa N, et al. (2003) Relationships between S-100 protein-positive cells and clinicopathological factors in patients with colorectal cancer. Anticancer Res 23:4423–4426
Deitch EA, Winterton J, Li M, et al. (1987) The gut as a portal of entry for bacteremia: role of protein malnutrition. Ann Surg 205:681–692
Penn RL, Maca RD, Berg RD (1985) Increased translocation of bacteria from the gastrointestinal tracts of tumor-bearing mice. Infect Immun 47:793–798
Kale IT, Kuzu MA, Berkem H, et al. (1998) The presence of hemorrhagic shock increases the rate of bacterial translocation in blunt abdominal trauma. J Trauma 44:171–174
Choudhry MA, Ren X, Romero A, et al. (2004) Combined alcohol and burn injury differentially regulate P-38 and ERK activation in mesenteric lymph node T cell. J Surg Res 121:62–68
Margaritis VG, Filos KS, Michalaki MA, et al. (2005) Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg 29:1329–1334
Ono S, Tsujimoto H, Yamauchi A, et al. (2005) Detection of microbial DNA in the blood of surgical patients for diagnosing bacterial translocation. World J Surg 29:535–539
Shimizu T, Tani T, Endo Y, et al. (2002) Elevation of plasma peptidoglycan and peripheral blood neutrophil activation during hemorrhagic shock: plasma peptidoglycan reflects bacterial translocation and may affect neutrophil activation. Crit Care Med 30:77–82
Shimizu T, Tani T, Hanasawa K, et al. (2001) The role of bacterial translocation on neutrophil activation during hemorrhagic shock in rats. Shock 16:59–63
Kanwar S, Windsor AC, Welsh F, et al. (2000) Lack of correlation between failure of gut barrier function and septic complications after major upper gastrointestinal surgery. Ann Surg 231:88–95
Sedman PC, Macfie J, Sagar P, et al. (1994) The prevalence of gut translocation in humans. Gastroenterology 107:643–649
Shimizu K, Ogura H, Goto M, et al. (2006) Altered gut flora and environment in patients with severe SIRS. J Trauma 60:126–133
Deitch EA, Xu D, Kaise VL (2006) Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci 11:520–528
Beal AL, Cerra FB (1994) Multiple organ failure syndrome in the 1990s: systemic inflammatory response and organ dysfunction. JAMA 271:226–233
Haga Y, Beppu T, Doi K, et al. (1997) Systemic inflammatory response syndrome and organ dysfunction following gastrointestinal surgery. Crit Care Med 25:1994–2000
Deitch EA, Xu DZ, Qi L, et al. (1991) Bacterial translocation from the gut impairs systemic immunity. Surgery 109(Pt 1):269–276
Berg RD, Wommack E, Deitch EA (1988) Immunosuppression and intestinal bacterial overgrowth synergistically promote bacterial translocation. Arch Surg 123:1359–1364
Tani T, Hanasawa K, Endo Y, et al. (1997) Bacterial translocation as a cause of septic shock in humans: a report of two cases. Surg Today 27:447–449
Kavanaugh MJ, Clark C, Goto M, et al. (2005) Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns 31:290–296
Acknowledgment
The authors thank Dr. Yasuaki Ueda and Dr. Seishirou Inaba (Department of Surgery, Nara City Hospital) for furnishing clinical data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiomi, H., Shimizu, T., Endo, Y. et al. Relations Among Circulating Monocytes, Dendritic Cells, and Bacterial Translocation in Patients With Intestinal Obstruction. World J Surg 31, 1806–1812 (2007). https://doi.org/10.1007/s00268-007-9110-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-007-9110-7