Abstract
Background
QueryHyaluronic acid (HA) is a large polymer increasingly used as dermal filler. HA does not permeate through healthy skin and is administered using various injection techniques. As HA procedures become more popular, the number of complications in facial rejuvenation procedures is likely to increase. Alternative methods may be necessary to satisfy the increasing demand for HA procedures. High-frequency high-intensity ultrasound is a painless and noninvasive method to deliver large molecules to the skin that is expected to deliver HA with visible results.
Objective
Assess facial rejuvenation with HA delivered with high-frequency high-intensity ultrasound.
Methods
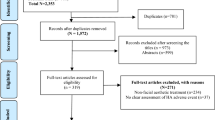
Fifteen women (mean age 55) willing to participate in a randomized, double-blind, face-split trial with HA and placebo formulations in different sides of the face, were subject to five treatment sessions with high-frequency high-intensity ultrasound. Photographs taken before the procedure and after the last procedure were evaluated by a panel of five experts, blind to which side was treated with the HA or with the placebo.
Results
The expert panel identified a noticeable facial rejuvenation in the HA side relative to the placebo with a very statistically significant difference between the two sides (p < 0.0001).
Conclusions
Administration of HA with high-frequency high-intensity ultrasound is safe and leads to unambiguous facial rejuvenation.
Level of Evidence I
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.





Similar content being viewed by others
References
Stern R, Maibach HI (2008) Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol 26:106–122
Meyer LJM, Stern R (1994) Age-dependent changes of hyalutonan in human skin. J Invest Dermatol 102:385–389
Oh J-H, Kim YK, Jung J-Y, J-e Shin, Kim KH, Cho KH et al (2011) Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci 62:192–201
Sakai S, Yasuda R, Sayo T, Ishikawa O, Inoue S (2000) Hyaluronan exists in the normal stratum corneum. J Invest Dermatol 114:1184–1187
Chiang YZ, Pierone G, Al-Niaimi F (2017) Dermal fillers: pathophysiology, prevention and treatment of complications. J Eur Acad Dermatol Venereol 31:405–413
Lee J-H, Kim S-H, Park E-S (2017) The efficacy and safety of HA IDF plus (with lidocaine) versus HA IDF (without lidocaine) in nasolabial folds injection: a randomized, multicenter, double-blind, split-face study. Aesth Plast Surg 41:422–428
Succi IB, da Silva RT, Orofino-Costa R (2012) Rejuvenation of periorbital area: treatment with an injectable nonanimal non-crosslinked glycerol added hyaluronic acid preparation. Dermatol Surg 38:192–198
Pugh WJ, Roberts MS, Hadgraft J (1996) Epidermal permeability—penetrant structure relationships: 3. The effect of hydrogen bonding interactions and molecular size on diffusion across the stratum corneum. Int J Pharm 138:149–165
Magnusson BM, Anissimov YG, Cross SE, Roberts MS (2004) Molecular size as the main determinant of solute maximum fux across the skin. J Invest Dermatol 122:993–999
Dragicevic N, Maibach HI (2017) Preface. In: Dragicevic N, Maibach HI (eds) Physical methods in penetration enhancement. Spinger, Heidelberg, p v
Sa GFF, Serpa C, Arnaut LG (2013) Stratum corneum permeabilization with photoacoustic waves generated by piezophotonic materials. J Control Resease 167:290–300
Sa GFF, Serpa C, Arnaut LG (2017) Photoacoustic waves as a skin permeation enhancement method. In: Dragicevic-Curic N, Maibach HI (eds) Physical methods in penetration enhancement. Springer, Heidelberg, pp 175–191
Bommannan D, Menon GK, Okuyama H, Elias PM, Guy RH (1992) Sonophoresis. II. Examination of the mechanism(s) of ultrasound-enhanced transdermal drug delivery. Pharm Res 9:1043–1047
Park HK, Kim E, Kim J, Ro Y, Ko J (2015) High-intensity focused ultrasound for the treatment of wrinkles and skin laxity in seven different facial areas. Ann Dermatol 27:688–693
Acknowledgements
The authors wish to thank Dr. Gonçalo Sá and Ângela Correia for assistance with the ex vivo studies. Funding was provided by Fundação para a Ciência e a Tecnologia (Grant No. 007630UID/QUI/00313/2013), H2020 Research Infrastructures (Grant No. LASERLAB EUROPE 654148).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
LGA holds intellectual property rights and equity related to the technology for active dermal filler administration. MJFS and RC declare that they have no conflicts of interest.
Ethical Standards
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Rights and permissions
About this article
Cite this article
Freire dos Santos, M.J., Carvalho, R. & Arnaut, L.G. Split-Face, Randomized, Placebo-Controlled, Double-Blind Study to Investigate Passive Versus Active Dermal Filler Administration. Aesth Plast Surg 42, 1655–1663 (2018). https://doi.org/10.1007/s00266-018-1208-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-018-1208-9