Abstract
Sexual coercion, in the form of forced copulations, is relatively frequently observed in orangutans and generally attributed to their semi-solitary lifestyle. High ecological costs of association for females may be responsible for this lifestyle and may have prevented the evolution of morphological fertility indicators (e.g., sexual swellings), which would attract (male) associates. Therefore, sexual conflict may arise not only about mating per se but also about associations, because males may benefit from associations with females to monitor their reproductive state and attempt to monopolize their sexual activities. Here, we evaluate association patterns and costs for females when associating with both males and females of two different orangutan species at two study sites: Suaq, Sumatra (Pongo abelii), and Tuanan, Borneo (Pongo pygmaeus wurmbii). Female association frequency with both males and females was higher in the Sumatran population, living in more productive habitat. Accordingly, we found that the cost of association, in terms of reduced feeding to moving ratio and increased time being active, is higher in the less sociable Bornean population. Males generally initiated and maintained such costly associations with females, and prolonged associations with males led to increased female fecal cortisol metabolite (FCM) levels at Tuanan, the Bornean population. We conclude that male-maintained associations are an expression of sexual conflict in orangutans, at least at Tuanan. For females, this cost of association may be responsible for the lack of sexual signaling, while needing to confuse paternity.
Significance statement
Socioecological theory predicts a trade-off between the benefits of sociality and the ecological costs of increased feeding competition. Orangutans’ semi-solitary lifestyle has been attributed to the combination of high association costs and low predation risk. Previous work revealed a positive correlation between association frequencies and habitat productivity, but did not measure the costs of association. In this comparative study, we show that females likely incur costs from involuntary, male-maintained associations, especially when they last for several days and particularly in the population characterized by lower association frequencies. Association maintenance therefore qualifies as another expression of sexual conflict in orangutans, and especially prolonged, male-maintained associations may qualify as an indirect form of sexual coercion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most mammals, female reproductive success is limited by access to food resources, while that of males is mainly limited by access to females (Darwin 1871; Emlen and Oring 1977). Hence, males and females have different behavioral strategies to optimize their lifetime fitness, which may lead to sexual conflict (Trivers 1972; Parker 1979). The high male-biased operational sex ratios in species with long lactational infertility and no paternal care may exacerbate sexual conflict (Clutton-Brock and Parker 1992; van Schaik 2016). Orangutan (Pongo spp.) females exhibit the longest inter-birth intervals in primates of 6 to 9 years (van Noordwijk et al. 2018), males do not provide direct paternal care for infants (Rijksen 1978; Utami Atmoko et al. 2009), and males are not territorial (Spillmann et al. 2017). Hence, male-male competition for receptive females is high, which carries a high potential for sexual conflict (Trivers 1972; Parker 1979). However, the relative importance of male-male competition, female choice, and sexual conflict in orangutans remains incompletely understood (Nadler 1981; Fox 1998, 2002; Knott 2009; Utami Atmoko et al. 2009; Knott et al. 2010; Spillmann et al. 2010, 2017). There is evidence, however, for the behavioral expression of sexual conflict, in the form of frequent forced copulations (Galdikas 1985a; Mitani 1985; Schürmann and van Hooff 1986; Knott et al. 2010). Females are vulnerable to this form of sexual coercion because of the pronounced sexual dimorphism (Smuts and Smuts 1993), the semi-solitary lifestyle (Rijksen 1978; van Schaik 1999), and the absence of morphological fertility advertisements, such as sexual swellings (Nunn 1999; Zinner et al. 2004). Interestingly, apparent physical injuries resulting from forced copulations have not been reported, and males seem to use only as much force as is necessary to achieve intromission (Knott 2009).
Sexual conflict over associations
Sexual conflict may arise not only about actual mating but also about association maintenance. Female orangutans are at the solitary end of the fission-fusion spectrum (i.e., females spend on average 50–80% of their time with only their own dependent offspring; van Schaik 1999; van Noordwijk et al. 2009). The low association frequency suggests that the costs of association, in terms of increased scramble feeding competition and hence reduced energy acquisition, are substantial for both males and females (Galdikas 1988; van Schaik and Fox 1996; Utami Atmoko et al. 1997). Yet, associations occur nevertheless, even if only one partner benefits. Specifically, because of the rare siring opportunities, male association decisions may be less cost-sensitive (van Schaik 1999), and males may accept foraging costs due to increased copulation opportunities (orangutans: Mitani 1989; chimpanzees [Pan troglodytes]: Emery Thompson and Georgiev 2014; Georgiev et al. 2014). Therefore, males likely benefit from associations with females, as this presumably facilitates monitoring their reproductive status and sexual activities and may be attempts to mate guard females, even if females are often unlikely to be fertile. As a result, females and males may experience a conflict about associating with each other. In fact, females may attempt to reduce time spent in associations because of potential foraging costs (e.g., Knott et al. 2018), whereas males often attempt to prevent them from leaving (van Noordwijk and van Schaik 2009). If this is indeed the case, male-female associations in orangutans can be considered an expression of sexual conflict, and male-maintained associations could be seen as an indirect form of sexual coercion (cf. Muller et al. 2009).
Absence of fertility advertisement and the cost-of-sexual-attraction hypothesis
Female orangutans do not exhibit any apparent graded, morphological signals advertising fertility (e.g., sexual swellings; Nunn 1999; Zinner et al. 2004). Although rare observations of female proceptive copulations with dominant males have been linked to the peri-ovulatory period (Fox 1998; Knott et al. 2010), ovulation appears largely concealed, as males initiate copulations independent of the females’ reproductive state and during periods of lactational infertility (Nadler 1981; Knott et al. 2010; Kunz 2020). Unpredictable ovulation in other catarrhine primates has been linked to the need to counteract male monopolization and serves to confuse paternity and so offset the risk of infanticide (Hrdy 1979; van Noordwijk and van Schaik 2000; van Schaik et al. 2000, 2004). However, signaling fertility bears costs for females (Matsumoto-Oda 1998; Archie et al. 2014). Particularly, the energetic costs of grouping may have prevented females from evolving signals that advertise prolonged fertility and attract male associates (Slater et al. 2008; Emery Thompson et al. 2014), thereby achieving such paternity confusion. Accordingly, Wrangham (2002) developed the “cost-of-sexual-attraction” hypothesis to explain the variation in morphological fertility advertisement in the genus Pan. Indeed, the cost of association for females (i) is negatively correlated with the number of swelling cycles to conception (Deschner et al. 2004; Emery Thompson 2005; Douglas et al. 2016) and (ii) is positively correlated with the rate of sexual coercion and infanticide (Wilson et al. 2014). In a high-quality habitat, both the immediate and delayed benefits to associate with males and to signal fertility over an extended period may outweigh the costs for females. Bonobos (Pan paniscus) are at this low cost of association end (Furuichi 2011; Clay et al. 2016; Nurmi et al. 2018) and have very prolonged periods of sexual attractivity (Douglas et al. 2016), and sexual coercion by males is virtually absent (male aggression: Hohmann and Fruth 2003; Paoli 2009; infanticide: Hohmann et al. 2019). The cost of association for chimpanzee females varies geographically. In a more gregarious West African chimpanzee population (P. troglodytes verus; Boesch and Boesch-Achermann 2000; Riedel et al. 2011), rates of sexual coercion are low (Stumpf and Boesch 2010) and females may even directly profit from signaling prolonged sexual attractiveness (“Social Passport Hypothesis”; Deschner and Boesch 2007). In contrast, in an East African chimpanzee population (P. troglodytes schweinfurthii), females’ foraging effort is compromised and their energy balance decreases with an increasing number of males in association (Emery Thompson et al. 2014). Consistent with the cost-of-sexual-attraction hypothesis, East African chimpanzees have fewer swelling cycles per conception (Emery Thompson 2005; Deschner et al. 2004; but see Deschner and Boesch 2007), more male sexual coercion (Muller et al. 2007, 2011), and more infanticide (Wilson et al. 2014) than West African chimpanzees.
Following the idea of the cost-of-sexual-attraction hypothesis, the absence of morphological fertility advertisement in the genus Pongo may reflect the prohibitively high costs of association to repeatedly signal fertility, while still needing to avoid male monopolization and thus confuse paternity. Here, we evaluate the costs of association in orangutans; future studies will investigate how those relate to the frequency of sexual coercion.
Geographic variation in sociability in orangutans
In the absence of high predation pressure due to their arboreal life style (van Schaik and van Hooff 1983), food abundance is the major constraint to population density and sociality in orangutans (van Schaik 1999; Hardus et al. 2013; Vogel et al. 2015). Fruit availability is not only thought to be responsible for the higher association frequency in West Sumatran (Pongo abelii) (average daily party size ranging from 1.6 to 1.9 individuals) compared to both Eastern Sumatran (P. abelii) as well as Bornean orangutans (Pongo pygmaeus) (average daily party size ranging from 1.05 to 1.3 individuals) (van Schaik 1999; Mitra Setia et al. 2009; Wich et al. 2011; Roth et al. 2020), it also likely constrains associations within populations over time (van Schaik and Fox 1996; Fox 1998; Wich et al. 2006; Roth et al. 2020; J.Meric de Bellefon et al., unpublished data). A high degree of scramble competition has been held responsible for the low female sociability, and direct female-female contest competition has also been reported (Utami Atmoko et al. 1997; Knott et al. 2008; van Noordwijk et al. 2012; Marzec et al. 2016). The ecological effect on association frequency, however, would be expected to be most prominent in associations between males and females because of the sex differences in ranging patterns and activity budgets connected to their distinct energetic demands (Morrogh-Bernard et al. 2009; van Schaik et al. 2009; Harrison et al. 2010; Vogel et al. 2017). In previous studies, it has been shown that day journey length increases with increasing association size, indicative of increased scramble competition both in Sumatran and Bornean populations (Fox 1998; van Schaik 1999; Wartmann et al. 2010).
In some respects, it appears that the orangutan species have adapted to their distinct conditions, and even under similar food availability in captivity they show a different response to increased sociability. In zoos, Bornean orangutans permanently housed with up to 5 adults exhibited overall higher fecal cortisol metabolite (FCM) levels than the more gregarious Sumatran orangutans living in groups with up to 8 adults, which was attributed to species differences in the sensitivity to social stress (Weingrill et al. 2011). Moreover, captive Bornean orangutans that were kept in fission-fusion like housing systems exhibited lower FCM levels than those kept in a stable group (Amrein et al. 2014). Taken together, these results indicate that social factors, especially extended sociality, lead to a stronger physiological stress response in the less sociable Bornean orangutans, suggesting that they will also prefer lower association rates in the wild.
Male bimaturism and sexual conflict
Orangutans exhibit a uniquely pronounced male bimaturism, which has been associated with alternative male reproductive strategies (MacKinnon 1974; Utami Atmoko and van Hooff 2004; Pradhan et al. 2012; Dunkel et al. 2013). Unflanged males, who lack secondary sexual characteristics, reportedly associate, copulate, and coerce copulations more frequently than flanged males in the majority of study populations (MacKinnon 1974; Galdikas 1985b; Sugardjito et al. 1987; Knott 2009; Mitra Setia et al. 2009; Utami Atmoko et al. 2009; JAK et al., unpubl. data). Flanged males, who have fully developed secondary sexual characteristics, emit long calls and are reported to rely largely on female choice around conception (Fox 2002; Mitra Setia and van Schaik 2007; Spillmann et al. 2010). Although there is evidence for variation among populations and species in the reproductive strategies of the male morphs (Delgado and van Schaik 2000; Knott and Kahlenberg 2007; Mitra Setia and van Schaik 2007; Utami Atmoko et al. 2009; Spillmann et al. 2017), sexual conflict over associations is likely more pronounced with unflanged males than with flanged males, because the former associate more frequently with females and cannot rely on female choice.
Aim of the study
Associations and their maintenance may present another, more subtle, context of sexual conflict in addition to forced copulations in orangutans. Following the “cost-of-sexual-attraction” hypothesis (Wrangham 2002), high costs of association may be responsible for the absence of morphological fertility advertisements in female orangutans. Here, we evaluate the costs of association for female orangutans with both females and males at two study sites, Suaq (P. abelii, Sumatra) and Tuanan (P. pygmaeus, Borneo) using behavioral and endocrine data. Because of large within-species variation in terms of their socioecology (e.g., Vogel et al. 2015; Roth et al. 2020) and little evidence for life history differences between species (van Noordwijk et al. 2018), we refer to study site rather than species differences, as we evaluate only one study site per species. We included female-female associations as a comparative category with the assumption that females have similar incentives to associate with each other (van Noordwijk et al. 2012) as opposed to male-female associations and that these therefore are likely cost-sensitive (sensu van Schaik 1999). We measured behavioral changes (i.e., changes in the daily activity budget) and variation in fecal cortisol metabolite (FCM) levels of parous females in relation to different types of association and social interactions. We hypothesized that (1) if males benefit from monitoring a female’s reproductive state and potentially attempt to monopolize a female’s sexual activities, they likely initiate and maintain such associations with females; (2) social interactions between males and females are rare and therefore females likely do not gain direct social benefits from associations with males (for an exception see Marzec et al. 2016), whereas associations with other females may provide social benefits for their infants (e.g., play opportunities; van Noordwijk et al. 2012); (3) associations with both males and females lead to higher foraging costs, i.e., increased moving and reduced feeding time, for females of the less sociable population, Tuanan, than females of the more sociable population, Suaq; and (4) besides scramble competition, costs of grouping females may bear additional costs from agonistic interactions, especially the occurrence of forced copulations, during associations with males. In Table 1, we provide a detailed overview of hypotheses and predictions.
Methods
Study sites and study subjects
Behavioral focal data on individually recognized adult females were collected at the long-term field sites of Tuanan, Mawas Reserve, Central Kalimantan, Indonesia (02° 15′ S; 114° 44′ E) and Suaq, Gunung Leuser National Park, South Aceh, Indonesia (03° 02′ N; 97° 25′ E) between July 2003–July 2018 and June 2007–March 2018, respectively. Because parous females are in continued association with their dependent offspring (van Noordwijk et al. 2009) and lactate over multiple years (van Noordwijk et al. 2013), both association patterns and the cost-benefit balances incurred by sociability are likely different from nulliparous (adolescent) females (van Schaik et al. 2009; Ashbury et al. 2020). Therefore, only data on parous females with a dependent offspring were included in this study (N = 20 females [Suaq: 6; Tuanan: 14]). Similarly, females who had lost their infants due to unknown reasons (Marzec et al. 2016; MAvN et al. unpubl. data) were excluded from the analyses after the loss of their infants, until they had given birth to a new infant. Infant loss is an extremely rare event (van Noordwijk et al. 2018), and insufficient data were available to add them as a separate category. The age of the dependent offspring of females was taken as a proxy for their reproductive state and included in all the analyses (Table 2). Infant ages were either known because the birth was directly observed or estimated from the first time an infant was observed (Table 2; cf. van Noordwijk et al. 2018).
Behavioral data
Activity budget
Behavioral data were collected according to an established, standardized protocol (https://www.aim.uzh.ch/de/orangutannetwork/sfm.html). We collected 2-min instantaneous data during full-day female focal follows on their activities (feeding, moving, resting, and social interactions). We recorded all occurrences of any individual in association (within 50-m distance) per 2-min interval and ad libitum social interactions with the focal individual. Social partners included the female’s own dependent and independent offspring, adolescent individuals, and adult females and males (unflanged and flanged). We subdivided social interactions into sexual, affiliative, and aggressive interactions. Sexual interactions comprised genital investigations by males, copulations, and copulation attempts. Copulations were labelled as either forced, if the female showed any resistance behavior (e.g., repeated attempt to move away, struggling against the males attempt to intromit), or unforced (following the definition of Fox 1998). We grouped aggression between females and both males and other females (excluding forced copulations) into non-physical (displays, short chases) and physical aggression (fights or coercive hand holding by males (van Schaik et al. 2006)). Affiliative interactions comprised allo-grooming, touching another individual, sitting in body contact, and begging for and sharing food. Because focal animals were individually recognized, we could not collect blinded data. Only data from well-trained observers with high inter-observer reliability were included in the analyses.
Association patterns and social interactions
Male focal follows collected with the same methods as the female focal follows were used to enhance the data set on the duration of associations and a fuller record of male-female dyadic interactions resulting in 960 male-female associations (Suaq: 292; Tuanan: 668) with known start and end times. An association between two individuals could last for multiple days and contain breaks, i.e., the association partners were at a distance of more than 50 m. Orangutans most likely perceive the presence of other individuals at distances of more than 50 m better than humans on the ground (van Noordwijk et al. 2009, 2012), and therefore, brief “separations” (> 50-m distance) likely are not relevant to them. If breaks lasted for longer than one full-day focal follow, we considered it as two separate association units. We recorded the individual responsible for any distance changes (in distance classes: contact, no contact < 2 m, 2–5 m, 5–10 m, 10–50 m) during the association, as well as the initiator (first approach to < 50 m) and terminator (who left to > 50 m) of associations. We calculated the Female Hinde Index (FHI) for female-male associations based on these approaches and leaves as follows:
A positive FHI indicates that the female was on average responsible for the maintenance of the association, while a negative FHI stands for a male-maintained association (Hinde and Atkinson 1970). The FHIs were calculated over all known approach and leave events and distance classes per association. Detailed approach and leave data throughout the association for the FHIs is available for 665 male-female associations (Suaq: 223; Tuanan: 442).
Ecological data
The monthly Fruit Availability Index (FAI; percentage of trees with fruits over all surveyed trees) was obtained from monthly phenology surveys of ~ 1500 trees at Tuanan and ~ 1000 trees at Suaq (Harrison et al. 2010; Vogel et al. 2015) (Table 3). Because the FAI is generally higher at Suaq than at Tuanan (Wich et al. 2011), we z-transformed all the FAIs within study site prior to the analyses (zFAI) to assess local FAI effects rather than between site comparisons. Although the FAI does not include fruits from lianas, which are components of orangutan diet, it corresponds well to the total proportion of fruits, i.e., high-quality food items, in their diet (Vogel et al. 2017), and can thus be taken as a proxy for forest productivity (Vogel et al. 2015).
Endocrine data
Collection, preservation, and extraction of fecal samples
We measured fecal cortisol metabolite (FCM) levels for females during association and non-association days. Fecal material was collected non-invasively, when individuals defecated naturally. Because there is an excretion lag time of 24–72 h for fecal cortisol metabolites (Weingrill et al. 2011), samples were collected on at least 5 consecutive days, once a day, preferably in the morning. Due to individual ranging patterns in orangutans, samples could only be taken during focal follows lasting 5–10 days with at least 5 weeks between successive sample periods, because individuals were not followed during this time. The methods to preserve and extract fecal samples from orangutans for hormone analyses have been established and validated (Weingrill et al. 2011; Amrein et al. 2014; Marty et al. 2015; Nugraha et al. 2016). Because of logistic constraints and varying infrastructures at the two field sites, different preservation and extraction methods had to be used. Generally, the fresh feces were homogenized using a stick and a 2–5-g aliquot was collected for analysis. Only samples not contaminated with urine were taken. When electricity from solar power was available, the fresh feces were collected into a polypropylene tube and frozen at − 18 °C upon return to the field station in the evening. All samples remained frozen until transported to the endocrinology laboratory at Bogor Agricultural University where samples were lyophilized, pulverized and subsequently extracted with 80% methanol in water as described in detail elsewhere (Weingrill et al. 2011; Nugraha et al. 2016). At Suaq and when electricity supply was not guaranteed at Tuanan, fecal samples were placed in a tube containing 5 ml of 80% ethanol in water for preservation upon collection. Samples were extracted upon return to the field station using a field-friendly, previously validated extraction method (Nugraha et al. 2016). Although these extraction methods have been shown to produce results which are strongly correlated (Nugraha et al. 2016), we controlled for potential extraction method differences by normalizing all FCM measurements within individual and method using z-transformations in the statistical analyses (van de Pol and Wright 2009; for details see data analysis section).
Hormone measurement
Fecal cortisol metabolite levels were measured in a total of 745 samples (Table 2) using a microtiter plate enzyme immunoassay (EIA) for 11ß-hydroxyetiocholanolone (Ganswindt et al. 2003), a major metabolite of cortisol in primate feces (Heistermann et al. 2006). The assay has been previously validated and successfully applied for assessing adrenocortical activity in numerous primate species (e.g., Heistermann et al. 2006) including captive and wild orangutans (Weingrill et al. 2011; Amrein et al. 2014; Marty et al. 2015). Samples used for this study were analyzed in different cohorts at two different laboratories (German Primate Center, DPZ, by A. Heistermann and Bogor Agricultural University, IPB, by JAK), with the locality of analysis for each sample included as a fixed effect in the statistical analyses (results remain the same if we standardize by both laboratory, method and individual, and are not shown below). EIAs were performed as previously described (Heistermann et al. 2004). Samples from the same individual were analyzed on the same microtiter plate, whenever possible. Each sample was analyzed in duplicate. We remeasured samples with a coefficient of variation (CV) > 7% between duplicates. Moreover, we reran any microtiter plate for which the intra-assay CV of the internal high- and low-value quality controls exceeded 10%. For the samples analyzed at both IPB and DPZ, the intra-assay CVs were below 10%, and the inter-assay CVs did not exceed 15%. All FCM concentrations are expressed in ng/g dry fecal weight.
Statistical analyses
All the statistical analyses were conducted in R version 3.5.2 (R Core Team 2018). We ran (generalized) linear mixed effect models ([G]LMM) using the “lme4” and “lmerTest” packages (Bates et al. 2015; Kuznetsova et al. 2017). Model assumptions (normality [for LMMs], homoscedasticity) were checked by the visual inspection of residual plots. Variance Inflation Factors (VIF) were calculated to examine potential multi-collinearity issues using the “car” package (VIF < 2, for the full model without interaction terms included and VIF < 4 for the full model with interaction terms) (Fox and Weisberg 2018). Further, we checked all the models for influential cases and outliers (Cook’s distance from the package “influence.ME” by Nieuwenhuis et al. 2012). The P value of 0.05 was used as a cutoff value for significance. For all statistical analyses, full models including all variables (social, ecological, and physiological factors) and their possible 2nd-order interactions (if applicable) were set up, and compared to the control model, containing all the random and control (ecological and physiological) factors, using likelihood ratio tests. All figures were generated using the “ggplot2” (Wickham 2016) and “cowplot” (Wilke 2019) packages.
Behavioral data—association patterns and maintenance
We evaluated the time (average daily hours) females spent in association with either other females or unflanged and flanged males during follow periods in separate analyses (LMM) and with the study site, zFAI, and the age of the dependent offspring as fixed effects. Individual identity was added as a random intercept.
We assessed when associations were male-maintained by setting up a binomial GLMM based on the FHI values (male-maintained when FHI < 0). We added study site, male morph, the age of the dependent offspring of the female (years), local fruit availability (zFAI), the occurrence of copulations (both unforced and forced), and association duration as fixed effects. To account for having the same individuals in several association dyads, both female and male identities were added as crossed random intercepts.
We formulated a Cox proportional hazard mixed model (survival analysis) using the package “coxme” (Therneau 2018) to evaluate if male-female associations lasted over more consecutive days than female-female associations based on the female focal follow data. We used right-censored data to account for unknown association endings, because females were no longer followed despite still being in association (N = 625 associations [Suaq: 61 associations with females, 53 with flanged males and 173 with unflanged males; Tuanan: 81 with females, 96 with flanged males, 161 with unflanged males] during 167 female FPs and of 21 females). We included both associations with known and unknown start times, because excluding associations with unknown start times (~ 40% of association dyads) would have introduced a bias against long associations in the analysis (for further details on this issue and for the results excluding associations with unknown start time, see supplementary mat). Besides the type of adult association partners (female, unflanged and flanged male), we added study site, zFAI, and the age of the dependent offspring (years) as fixed effects in the model. We set contrasts for the association partner type to first compare association maintenance between male and female association partners and then the two male morphs. Further, we included the follow period nested in the female identity as a random intercept to avoid pseudo-replication.
Behavioral data—activity budget changes
The daily activity budget was calculated from the 2-min instantaneous data taken during full-day female focal follows (N = 2086; Suaq: 221; Tuanan: 1865), and thus, (1) it includes the association record over the entire day, and (2) it accounts for activity budget variation over daytime. To account for variation in activity budgets (van Noordwijk et al. 2012), we only included female follow periods (FP) that contained at least 5 full-day focal follows (mean = 8.3 ± SE 0.1) within 40 days (on average within 9 days) in the analyses. First, we evaluated the variability of the total active time, which comprised the total hours from leaving the morning nest to entering the evening nest. Second, we evaluated if female foraging behavior changed on days with associations and social interactions by analyzing variation in daily feeding time while controlling for moving time (offset term) (henceforth referred to as F:M ratio). Daily moving hours correlate strongly with day journey length (DJL) (Pearson correlation for available Tuanan data: R2 = 0.76, t769 = 32.66, P < 0.0001, N = 771 female full-day follows). We analyzed daily moving hours rather than DJL, because it is a proxy for daily travel, but also includes moves within feeding patches, with no net displacement in space, which are likely not accurately reflected in DJL. We tested for the effects of social, physiological and ecological factors on active time and F:M ratio in linear mixed models. We built in the female follow period (FP) nested in female identity as random intercepts to avoid pseudo-replication. The separate analyses on the changes of all activity budget components (feeding, resting, and moving hours) including separate analyses for each study site are reported in the supplementary materials (STable 11–14).
As social factors, we included the total cumulative time spent with either males or females and any agonistic and sexual interaction recorded as fixed effects (details in Table 3). Because consecutive association days are likely inter-dependent and there may be compensatory effects, we also included the total number of (known) consecutive days in association with either males or females in the full model. Further, we controlled for potential confounding physiological and ecological factors, overarching site differences (Tuanan, Suaq), and the total time spent in social interactions with any partner (Table 3). We tested for interaction terms between study site and social factors to check for population differences. Interaction terms were only included in the final model if they improved the model fit based on likelihood ratio tests. Both control models – including study site, zFAI, age of the dependent offspring and total social interaction time – significantly improved the null models, containing only the random intercepts (and the offset term) (active time: χ24,8 = 51.75, P < 0.001; F:M ratio: χ24,8 = 53.08, P < 0.001). We excluded 16 days when females fed less than 1 h and their active time was below 6 h because of serious health issues or lack of habituation, as these days revealed to be influential cases and the model assumptions were violated (for one context of these outliers see Marzec et al. 2016).
Endocrine data
The behavioral reference day corresponding to the measured FCM level was obtained by backdating 3 days from the date of collection of morning fecal samples and 2 days for samples collected after 2 pm (Cadilek 2009; Weingrill et al. 2011; Amrein et al. 2014; Nugraha et al. 2016). If there were several fecal samples for one behavioral reference day, only the morning sample was included in the final analysis. FCM levels with the same behavioral reference day were strongly correlated (r = 0.73, CI = [0.60, 0.83], P < 0.0001, N = 78). To control for any sample hour bias, the time of sample collection (i.e., time of defecation) was included in the statistical analyses as a control factor although previous data on captive-housed animals showed no time-of-day effect (Weingrill et al. 2011). FCM levels were ln-transformed to normalize their distribution. Subsequently, the values were standardized within individual and extraction method used, to be able to assess FCM level changes caused by social and ecological stressors within individuals rather than between individuals (method described in van de Pol and Wright 2009). Because such z-transformations may be sample size dependent, we only included those individuals in the analyses for which more than 10 samples and at least 5 known behavioral reference days for a given extraction method were available. The within-individual transformations were done including all available samples, including the samples without behavioral reference (N = 745). The analysis included only the samples with a known behavioral reference day (N = 370). The number of total samples available (per method and individual) was included in the analysis as a control factor. A linear mixed effect model (LMM) was set up to test for the effect of social factors on female FCM levels. The same social factor categories as described in the activity budget analyses were tested. The time to sample extraction (days), the total number of days an individual was followed, the age of the dependent infant (years), an activity budget parameter (feeding proportion), and the Fruit Availability Index (FAI) were included in the full model to control for possible confounding factors leading to FCM changes. The female follow period was added as a random intercept to avoid pseudo-replication. Because the FCM levels were standardized within individual and method, these two factors were not included as random intercepts in the analysis to keep the models as parsimonious as possible. The analyses without the standardization procedure and including individual identity and extraction method as random intercepts yielded the same patterns and are reported in the supplementary materials. The control model with all the potential confounding factors did not improve the model fit of the null model containing the random intercept term only (χ23,12 = 6.96, P = 0.64, ΔAIC = 11.04).
Results
Initiation and maintenance of associations
Association frequency
Despite substantial day-to-day variation, females spent on average (mean) 30.0 ± SE 0.1 min per day in association with other females (Suaq: 82.6 ± SE 13.6 min; Tuanan: 23.7 ± SE 2.4 min), 53 ± SE 0.1 min with unflanged males (Suaq: 200.1 ± SE 20.0 min; Tuanan: 35.1 ± SE 3.1 min), and 20 ± SE 0.0 min with flanged males (Suaq: 29.1 ± SE 6.8 min; Tuanan: 19.2 ± SE 2.3 min) (SFig. 1). The time females spent in association with both flanged and unflanged males increased as the age of their dependent offspring increased (flanged: β = 0.448 ± 0.090, t = 5.001, P < 0.001; unflanged: β = 0.606 ± 0.126, t = 4.799, P < 0.001; STable 1; SFig. 1), while the association time with other parous females remained constant with offspring age (β = 0.067 ± 0.063, t = 1.066, P = 0.29; STable 1; SFig. 1). Time spent in association with other parous females and unflanged males was generally higher at Suaq than at Tuanan (females: β = − 1.058 ± 0.360, t = − 2.939, P = 0.008; unflanged: β = − 1.821 ± 0.556, t = − 3.273, P = 0.003; STable 1; SFig. 1), but not with flanged males (β = 0.228 ± 0.332, t = 0.688, P = 0.5). To sum up, females were more frequently in association with unflanged males than with adult females or flanged males, partly supporting prediction 1.1 (Table 1), and time in association with both male morphs increased with the age of the dependent offspring of females (Table 1: prediction 3.2).
Association initiation and maintenance
Both flanged males (Tuanan: 82.1%; Suaq: 73.9%) and unflanged males (Tuanan: 84.0%; Suaq: 80.7%) initiated associations with females more frequently than the females themselves (Fig. 1). Moreover, both flanged males (Suaq: mean[FHI] = − 0.25 ± SE 0.06 [N = 63 associations]; Tuanan: − 0.38 ± SE 0.03 [N = 205]) and unflanged males (Suaq: − 0.16 ± SE 0.03 [N = 160]; Tuanan: − 0.12 ± SE 0.03 [N = 237]) maintained these associations (Fig. 2). The full model for the probability that associations were male-maintained explained significantly more variability than the null model (χ23,9 = 72.53, P < 0.0001, N = 665 of 30 female and 140 male identities; STable 2). First, especially long associations were more likely male-maintained (β = 1.226 ± 0.255, OR = 3.40, z = 4.804, P < 0.001). Second, flanged males were more likely to maintain associations with females than unflanged males (β = 0.653 ± 0.201, OR = 1.92, z = 3.244, P = 0.001), whereas this difference between male morphs was more pronounced at Tuanan than at Suaq, as the model fit marginally improved when adding this interaction term (χ29,10 = 4.12, P = 0.04). Association maintenance by males was independent of the female’s dependent offspring’s age (β = 0.089 ± 0.100, OR = 1.09, z = 0.891, P = 0.37), the local zFAI (β = − 0.019 ± 0.089, OR = 0.98, z = − 0.215, P = 0.83), and the occurrence of sexual interactions (β = 0.556 ± 0.298, OR = 1.74, z = 1.866, P = 0.06). In sum, prediction 1.2 (Table 1) was supported, as associations were male-initiated and male-maintained independent of the female reproductive state, whereas this was the case at both study sites and by both male morphs.
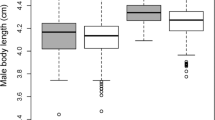
Female Hinde Index of associations with unflanged males (left) and flanged males (right) by study site (Suaq: top; Tuanan: bottom) by the age of the dependent offspring (year), as a proxy for female reproductive status. The black crosses indicate the weighted mean FHI (by the number of known approaches and leaves) and their transparency is relative to the number of associations included. Data points (Suaq: orange; Tuanan: blue) represent individual association units and only include known approaches and leaves (N = 665). The data point size is relative to the association duration and a horizontal jitter function was applied to the data points to make overlapping data points more visible
Association maintenance over multiple days
Male-female associations were maintained over more consecutive days at Tuanan (maximum 8 days) than female-female associations (maximum 4 days), whereas at Suaq this difference between the maintenance of male-female (maximum 11 days) and that of female-female (maximum 7 days) associations was less pronounced (Fig. 3; Table 4). Accordingly, the survival analysis on the probability of ending an association was significantly better when including the interaction between study site and partner type (β = − 0.234 ± 0.089, HR = 0.79, P = 0.009): Female-female associations ended sooner at Tuanan than male-female associations; at Suaq this difference was less pronounced (Fig. 3). We could not find any difference in association maintenance probability between the two male morphs (unflanged vs. flanged) (Table 4). Associations were maintained over more consecutive days with the increasing age of the dependent offspring of a female (β = − 0.169 ± 0.066, HR = 0.84, P = 0.01). The interaction between the age of the dependent infant and partner type did not improve the model fit (χ22 = 0.83, P = 0.66). Local zFAI did not have an effect on the association maintenance (Table 4). All in all, both unflanged and flanged males maintained associations with females over more consecutive days than females did with other females at Tuanan, the less sociable population, whereas we find no such difference at Suaq, the more sociable population, supporting prediction 1.3 (Table 1) and its site difference but not the male morph component.
Kaplan-Meier survival curve for the maintenance of associations over consecutive days at Suaq (a) and Tuanan (b) by the association partner type (color). The survival curve is based on the female focal data from follow periods also including the non-full-day focal follows (e.g., days when an individual was found) (N = 625 [Suaq: 287; Tuanan: 338] associations of 21 females and 168 different FPs). The left-censored data is indicated in crosses
Social interactions between males and females
Affiliative social interactions occurred in 6.0 ± SE 0.8% of all male-female associations (STable 5), always once or twice (mean 1.45 ± SE 0.11 occurrences) during the entire association (female-unflanged: 0.022−h [interactions per association hour] [Suaq]; 0.025−h [Tuanan]; female-flanged: 0.016−h [Suaq]; 0.011−h [Tuanan]). Male aggression towards females outside of the sexual context was observed in 7.4 ± SE 0.9% of all dyadic male-female associations. Physical aggression by males directed at females was rare (Suaq: in 1 out of 393 associations; Tuanan: 9 of 521 associations) and consisted exclusively of coercive hand holding (van Schaik et al. 2006). Flanged males were significantly more likely to direct non-physical aggression in the form of displays, displacements, or short chases towards females both at Tuanan (14.0 ± SE 2.2% [0.091−h]) and at Suaq (9.0 ± SE 2.6% [0.025−h]) than unflanged males (Tuanan: 6.6 ± SE 1.5% [0.030−h]; Suaq: 6.6 ± SE 1.5% [0.013−h]) (STable 6). At Suaq and Tuanan both forced and unforced copulations were more frequent during associations involving unflanged males (Suaq: 23.5 ± SE 2.6% [0.075−h] [forced: 17.3 ± SE 2.3% (0.052−h)]; Tuanan: 15.0 ± SE 2.1% [0.041−h] [forced: 7.3 ± SE 1.5% (0.021−h)]) than flanged males (Suaq: 5.7 ± SE 2.1% [0.014−h] [forced: 4.1 ± SE 1.8% (0.011−h)]; Tuanan: 6.2 ± SE 1.5% [0.018−h] [forced: 1.2 ± SE 0.7% (0.005−h)]) (for more details: Kunz 2020). Moreover, especially unflanged males at Tuanan frequently investigated the genitals of females during associations (female-unflanged associations: 27.4 ± SE 2.4% [Tuanan], 7.7 ± SE 1.9% [Suaq]; female-flanged associations: 1.3 ± SE 0.7% [Tuanan], 4.2 ± SE 2.1% [Suaq]). These genital investigations occurred independent of the female’s offspring age (for details see suppl. mat. STable 7; SFig. 3). In summary, both affiliative and agonistic social interactions were rare during male-female associations (predictions 1.4 + 1.5, Table 1), indicating that costs of association to females likely result from increased feeding competition rather than the accompanying social interactions. However, sexual interactions were on average the most frequent social interactions during male-female associations indicating male mating effort, thus supporting prediction 1.6 and 3.2 (Table 1).
Activity budget changes
Active time
Female active time on days without any association partners except for her dependent offspring was on average 10.8 ± SD 1.0 h (min 6.1 and max 13.1), whereas on days with female associates it increased to 11.4 ± SD 1.0 h (Suaq: 11.6 ± SD 0.8; Tuanan: 11.3 ± SD 1.0) and on days with males in association (independent of association duration) to 11.4 ± SD 1.0 h (Suaq: 11.5 ± SD 0.8; Tuanan: 11.3 ± SD 1.0) (suppl. mat. STable 9). Accordingly, in both study populations, female active time increased significantly with increased time in association with females (β = 0.084 ± 0.025, t = 3.428, P = 0.001) and with males (β = 0.068 ± 0.034, t = 2.004, P = 0.045) (Fig. 4a+d). Moreover, at Tuanan a female’s active time increased significantly on days with copulations whereas it did not at Suaq (suppl. mat. STable 10), as the significant interaction between study site and days with copulations indicates (β = 0.387 ± 0.158, t = 2.444, P = 0.02) (Fig. 5a). Active time further increased with increased number of consecutive days with males (β = 0.056 ± 0.024, t = 2.385, P = 0.02), the total time spent in social interactions with any social partner (β = 0.556 ± 0.155, t = 3.587, P < 0.001), and the local fruit availability (β = 0.095 ± 0.039, t = 2.427, P = 0.02). Interaction terms between site and any other social factors, except copulation occurrence, did not significantly improve the model fit. In sum, daily active time increased in both populations for females in associations, and at Tuanan on days with copulations, and accordingly, the model fit significantly improved when including social factors (χ28,16 = 56.27, P < 0.001, ΔAIC = 40.27; N = 2086 of 20 females and 279 FP; for the full model suppl. mat. STable 10).
Daily female activity budget changes (from left to right: active time (a, d), feeding (b, e), and moving (c, f) hours) depending on cumulative hours spent with males (a–c) and females (d–f) and by study site (round, orange: Suaq; triangles, blue: Tuanan). Each data point represents one full-day focal follow (N = 2086), the regression lines are the correlations between hours spent with males/females and activity hours and do not show model predictions. The shaded areas display 95% confidence intervals
Daily female activity budget changes (from left to right: active time (a), feeding (b), and moving (c) hours) depending on the occurrence of copulations and by study site (orange Suaq, blue Tuanan). The boxplots are based on median values of full-day focal follows (N = 2086) and do not show model predictions (the hinges extend to the first and third quantiles and the whiskers to the largest value, and lowest, respectively, at most 1.5*inter-quartile range. Data points beyond the end of whiskers are plotted individually [Wickham 2016])
Foraging behavior
Overall daily feeding time (F) decreased with both males and females in association, whereas moving (M) and resting time increased (Fig. 4; for detailed analyses see suppl. mat. STable 9, 11, 12). At both study sites, the F:M ratio (time spent feeding per unit moving time) of females decreased with increased association time with males (β = − 0.250 ± 0.053, t = − 4.698, P < 0.001), whereas it decreased significantly more at Tuanan with increased time with females in association compared to Suaq (β = − 0.333 ± 0.070, t = − 4.763, P < 0.001). Only consecutive days with females, but not with males, led to a further decrease in a female’s daily F:M ratio (Table 5). Furthermore, on days with copulations, the F:M ratio decreased significantly more at Tuanan than at Suaq (β = − 0.630 ± 0.246, t = − 2.557, P = 0.01). The full model for the F:M ratio including social factors was significantly better than the control model including ecological and physiological factors only (χ28,17 = 80.47, P < 0.001, ΔAIC = 62.47; N = 2086 of 20 females and 279 FP) (Table 5). In sum, female foraging behavior was negatively affected by associations with both females and males, with the effects being more pronounced for the Tuanan population, supporting predictions 2.1 and 2.1.1 (Table 1) that costs of association arise from scramble competition of grouping. Less support was found for predictions 2.2 and 2.2.1 proposing additional costs caused by (agonistic) social interactions (Table 1).
FCM levels
Female FCM levels increased with the number of consecutive days in association with a male (β = 0.136 ± 0.047, t = 2.870, P = 0.004; Fig. 6; Table 6), but not with females (β = − 0.082 ± 0.085, t = − 0.960, P = 0.34). None of the other social factors, including daily association time with either females or males and the occurrence of aggression, further improved the model fit. Accordingly, the control model containing all physiological and ecological factors was improved significantly when adding the number of consecutive days with males (χ212,13 = 7.30, P = 0.007, ΔAIC = 5.30). Although one particular female (Desy), who had male associations over a course of 9 days, appeared to be the main driver for this result, there still was a trend for consecutive days with males leading to elevated FCM levels when this female was excluded (β = 0.106 ± 0.056, P = 0.06, N = 333 of 89 follow periods; comparison to control model: χ212,13 = 3.47, P = 0.06).
Standardized FCM levels (z-ln [FCM concentration (ng/g)]) (y-axis) of females in response to consecutive association days with adult males (a) and to consecutive association days with adult females (b). A jitter function was added to the plot to visualize the overlapping data points (consecutive days are only integers). The black diamond-shaped points indicate the mean FCM levels with the error bar (SE) in black. Study sites indicated by round, orange: Suaq; triangles, blue: Tuanan
Because our FCM data set only contained data for at most 4 consecutive days of female-female associations, we restricted the data set to sample days of at most 4 consecutive male-female association days in a further analysis. Then, the effect of consecutive days in association with males on female FCM levels was no longer significant (β = 0.083 ± 0.061, P = 0.18, N = 361 of 96 follow periods). In sum, it appears that only prolonged male-female associations over more than four consecutive days lead to increased female FCM levels.
Discussion
Foraging costs of association
Because female reproductive success is generally directly linked to access to resources (chimpanzees: Emery Thompson et al. 2007; apes: Emery Thompson et al. 2008; Stumpf et al. 2008; orangutans: Knott et al. 2009), the energetic costs of association with conspecifics have been held responsible for the varying degrees of gregariousness across the orangutan geographic distribution (van Schaik 1999). The females in our study likely suffer energetically from associations (with both males and adult females): In both study populations, females increased the length of their active day, but their feeding time decreased, both absolutely (suppl. mat. STable 12) and relative to moving time. This reduction is not only a trade-off directly resulting from increased time spent in social interactions, because (1) we controlled for time spent in social interactions, and (2) in the more sociable Sumatran population with higher forest productivity, feeding time was less affected by time spent in association with females. Hence, the reduced F:M ratio and the increased active time can be taken as direct evidence for elevated scramble competition, indicating that associations incur energetic costs to females, whereas we only found limited evidence for costs resulting from (agonistic) social interactions. We can conclude that females modify their activity budgets when in association with both males and females, in patterns that are congruent with increased scramble competition. However, to more directly assess the energetic costs of sociality in orangutans, our measure of F:M ratio should be complemented by more accurate measures of actual energy intake and measures of energy balance, such as analysis of urinary C-peptide concentrations (e.g., Emery Thompson and Knott 2008).
Orangutan females likely do not gain direct benefits from associations with males, whereas males need associations with females to monitor their reproductive status. First, genital investigations by males and male-initiated sexual interactions were the most frequent social interactions observed during male-female associations, whereas affiliative social interactions were extremely rare. However, benefits for females by associating with certain (flanged) males, such as protection from harassing males, cannot conclusively be ruled out (Mesnick 1997; Fox 2002). Second, most associations were both male-initiated and male-maintained, regardless of female reproductive state, i.e., the age of the female’s dependent offspring, and females likely incurred costs from those involuntary associations as discussed above. When females are ready to conceive, however, they may actively seek the association with (dominant) flanged males (Fox 1998, 2002; Spillmann et al. 2010). With our analyses, we did not capture this short window around conception. We conclude that females and males are likely at odds about association maintenance. Accordingly, orangutan females have been reported to actively avoid male associates or try to end associations as rapidly as possible (Fox 2002; Mitra Setia and van Schaik 2007; Utami Atmoko et al. 2009; van Noordwijk and van Schaik 2009; Spillmann et al. 2010; Knott et al. 2018). Further investigations to understand how and if females attempt to avoid male associates have to be conducted, including the analysis of simultaneous ranging data. In sum, our study indicates that females incur costs from male-maintained associations, but no clear immediate benefits (albeit perhaps indirect ones: Kunz 2020), especially during the period of lactational infertility (~ 6.5 years [van Noordwijk et al. 2018]). These costs of involuntary associations may be relevant, because orangutan females’ reproductive success highly depends on the availability of resources (Knott et al. 2009), particularly in a less productive habitat (Wich et al. 2011).
Stress and association
Female FCM levels increased as they spent more days in association with males, but not with females. This social factor was the best and only predictor for FCM level changes. Thus, repeated days of increased active time and reduced F:M ratio led to a physiological stress response. Interestingly, this was not the case when in association with other females, because females can avoid lengthy associations with other females before associations become too costly. Conversely, males appear to profit from associations with females and they maintain associations over a longer time period than a female would. The behavioral data available support this conclusion: Female-female associations never lasted more than 4 consecutive days at Tuanan, where the F:M ratio decreased significantly more when in association with other females than at Suaq, while male-female associations could last up to 8 days. The elevated FCM levels of captive orangutan females when artificially confined to permanent association with males (Amrein et al. 2014) further support our hypothesis that increased sociality over an extended time period leads to a physiological stress response, especially in Bornean orangutans. The findings in captivity suggest that Bornean females show stress reactions to extended sociality even in the absence of reduced net energy intake, suggesting that in captivity increased FCM levels in females associated with males more likely reflect social rather than energetic stress. Although our endocrine data set is very limited, especially for the extended consecutive association days with males, we propose that only extended association periods with males lead to increased FCM levels as seen in captivity. However, whether these FCM elevations observed in our wild females are a response to the association itself or, alternatively, reflect energetic constraints due to the association-related decrease in feeding time and increase in active time is unclear. Future studies should generally aim at obtaining a more conclusive endocrine data set including larger sample sizes linked to consecutive association days.
Following the same line of argument, one would expect to find more pronounced FCM level changes in the less sociable Bornean orangutans in response to involuntary associations compared to Sumatran orangutans. Although we could not find any evidence for differences in FCM level changes between Suaq and Tuanan, our data set was very small for the Suaq population (N = 52 samples, a maximum of 6 [known] consecutive days in male-female association). Thus, the comparison should be repeated with a more extensive data set in the future. A difference in the physiological response to social stressors, including energy balances, may be expected in the light of the socioecological theory, because the degree of sociability between the two populations differs (this study; van Schaik 1999). Since our activity and feeding data indicate that both associations (with females) and social interactions are costlier to Tuanan females than to Suaq females, where fruit availability is generally higher (Wich et al. 2011), a stronger physiological stress response would be expected at Tuanan. Future studies are, however, needed to test this hypothesis and thus to evaluate whether females of the more sociable Sumatran orangutan may be more “stress-resistant” which could explain why there is less need for either behavioral or physiological mechanisms to avoid associations.
We found no evidence for differences in female FCM levels on days with any agonistic interaction with either males or females in the two populations. Even though days with copulations were characterized by increased active time and reduced F:M ratio at Tuanan, we found no evidence that male aggression, in particular sexual coercion (SFig. 7), imposed any additional costs, either as reduced feeding time or in elevated FCM levels. If these forced copulations are cost insensitive, they would not qualify as sexual coercion by the definition of Smuts and Smuts (1993) (“use by a male of force, or threat of force, that functions to increase the chances that a female will mate with him at a time when she is likely to be fertile, and to decrease the chances that she will mate with other males, at some cost to the female”), while prolonged, male-maintained associations would. However, the absence of a stress response does not exclude other costs of forced copulations, such as the limitation to the expression of female mating preferences. Indeed, the consistent attempts by females to escape from involuntary mating initiations (Fox 2002; Knott et al. 2010) suggest that females perceive resisted copulations as undesired rather than as a way to assess mate quality. For now, therefore, interpreting forced copulations as sexual coercion remains the most plausible explanation.
Since fecal cortisol metabolite levels represent an integrative measure of pooled endocrine activity over several hours or days (Hodges and Heistermann 2011), it is likely unsuited to detect short-term stress responses to a specific behavioral event. Forced copulations lasted on average 8.8 ±SD 7.2 min (Kunz 2020), and any stress response associated with this behavior is likely to be too short to be detected by our FCM measure. Urinary cortisol levels may thus be a more appropriate measure to assess whether particular social interactions induce more immediate elevations in cortisol production (e.g., Silk et al. 2013) as has been shown for chimpanzees (Muller et al. 2007; Emery Thompson et al. 2010). Further detailed studies, with a larger sample size and more immediate measures of cortisol levels from urine, are needed to examine whether female orangutans do indeed not show stress responses to forced copulations.
The male perspective
Both unflanged and flanged males are responsible for maintaining associations, independent of the females’ dependent offspring age (as a proxy for reproductive state), which supports the hypothesis that the males’ interest to associate exceeds that of the females (Table 1). Besides mating opportunities, these associations may be an attempt to monitor a female’s reproductive state and sexual activities. In the absence of any apparent signal of fertility (Nunn 1999), it remains uncertain how males assess female reproductive state, if at all. The genital investigations reported here may provide some olfactory information to males (cf. chimpanzee: Matsumoto-Oda et al. 2003; review: Drea 2015), but data are insufficient to know how and if these relate to sexual interactions (suppl. mat. STable 8). It is likely that males also incur energetic costs from associations and interactions with females (cf. East African chimpanzees [P. troglodytes schweinfurthii]; Emery Thompson and Georgiev 2014; Georgiev et al. 2014), and our unpublished data suggest this, too, for orangutan males. Thus, males may have a set of decision rules when and for how long to associate with certain females. Accordingly, the time in association with males increases with the increasing age of the dependent offspring of females (this study; van Schaik 1999), suggesting some type of reproductive benefits for males. More detailed analyses on the social context of associations will provide further insight into how males benefit from sociality with females.
It remains to be investigated if prolonged male-maintained associations should be labelled as a separate indirect form of sexual coercion or may even function as coercive mate guarding, i.e., “to constrain female promiscuity” (Muller et al. 2009). First, direct non-sexual aggression towards females by males is rare in orangutans (STable 6; SFig. 2) providing little evidence for any herding, punishment or sequestration (apart from the ten cases of coercive hand holding). However, anecdotal data suggest subtle sequestration attempts, in that males may try to influence females’ travel direction away from other males during associations (MAvN et al., unpubl. data). Second, copulations regularly occur in the presence of other, even more dominant, males (Fox 2002). (Coercive) mate guarding by males therefore appears to be an inefficient strategy, especially for subordinate, unflanged males. Third, although we found evidence for direct costs for females resulting from male-maintained associations, which indicates male coercion, we cannot rule out that females ultimately benefit indirectly from paternity confusion through those male-driven association patterns. Future studies are needed to evaluate the social contexts of associations.
Conclusion
Here, we report evidence for sexual conflict over associations in orangutans. We conclude that females incur costs from male-maintained associations, especially if those associations last multiple days. The costs include reduced feeding time and increased moving and resting time, which adds up to longer activity per day and thus shorter night rest. Furthermore, prolonged associations with males were associated with elevated FCM levels, whereas this was not the case for female-female associations which were usually much shorter. We suggest that the absence of morphological fertility advertisement in female orangutans may be explained by these costs of association, thus supporting the first prediction of the “cost-of-sexual-attraction” hypothesis (Wrangham 2002) for orangutans. The length of sexual attractivity negatively correlates with the cost of association for females in the genus Pan (Wrangham 2002). Orangutans fit into this fission-fusion continuum at the solitary end: They do not exhibit any morphological signal of fertility, arguably because this would attract too many competing males at once leading to a prolonged period of unacceptably high energetic costs for the females, in addition to the mere physiological costs associated with the swelling itself (for a review: Nunn 1999). On the contrary, female orangutans advertise non-availability with small labial swellings during pregnancy (Schultz 1938; Galdikas 1981), likely to reduce the costs of association as males refrain from maintaining associations and copulating with pregnant females exhibiting the labial swelling (only 2 out of 34 pregnancy matings were observed when females exhibited a pregnancy swelling, JAK et al. unpubl. data).
Yet, females of both Pan spp. and Pongo spp. exhibit unpredictable ovulation, albeit to varying extent (Nadler 1981; Deschner et al. 2004; Douglas et al. 2016), which has been linked to paternity confusion serving infanticide avoidance strategies (Hrdy 1979; Hrdy and Whitten 1987; van Schaik et al. 2004). The concealed ovulation in orangutans (Nadler 1981) may therefore also serve to reduce the risk of infanticide as it does in most other primates (Hrdy 1979; van Schaik et al. 2004). Female orangutans seem to vary their mate preferences with their reproductive status accordingly (Knott et al. 2010). However, evidence for infanticidal attacks by males remains indirect (Beaudrot et al. 2009; Knott et al. 2019; Scott et al. 2019) and infant mortality is generally extremely low (van Noordwijk et al. 2018), suggesting that male infanticide in orangutans is extremely rare compared to chimpanzees and that females employ efficient counterstrategies.
In a dispersed mating system with high costs of association, and where males generally drive association patterns as found here for orangutans, the lack of morphological fertility advertisement can be explained by the selection on the total concealment of ovulation. Given a risk of infanticide (Knott et al. 2010, 2019), females must achieve an optimum distribution of paternity assessments (van Schaik and Janson 2000; van Schaik et al. 2004) by removing as much information on female fertility status as possible. Accordingly, the absence of morphological fertility advertisement combined with the concealed ovulation in orangutans appears to be the result of a trade-off between the costs of association and the necessity for paternity confusion (van Schaik et al. 2004; Knott et al. 2010, 2019). Future work will have to elaborate on the details of this hypothesis.
Data availability
The main data sets generated and analyzed during the current study are available in the Harvard Dataverse repository, https://doi.org/10.7910/DVN/WXDVF6.
References
Amrein M, Heistermann M, Weingrill T (2014) The effect of fission–fusion zoo housing on hormonal and behavioral indicators of stress in Bornean orangutans (Pongo pygmaeus). Int J Primatol 35:509–528
Archie EA, Altmann J, Alberts SC (2014) Costs of reproduction in a long-lived female primate: injury risk and wound healing. Behav Ecol Sociobiol 68:1183–1193
Ashbury AM, Willems EP, Utami Atmoko SS, Saputra F, van Schaik CP, van Noordwijk MA (2020) Home range establishment and the mechanisms of philopatry among female Bornean orangutans (Pongo pygmaeus wurmbii) at Tuanan. Behav Ecol Sociobiol 74:42
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beaudrot LH, Kahlenberg SM, Marshall AJ (2009) Why male orangutans do not kill infants. Behav Ecol Sociobiol 63:1549–1562. https://doi.org/10.1007/s00265-009-0827-1
Boesch C, Boesch-Achermann H (2000) The chimpanzees of the Taï Forest: behavioural ecology and evolution. Oxford University Press, New York
Cadilek MJ (2009) Female-male association patterns, mating conflict and hormonal stress response in Bornean orangutan females (Pongo pygmaeus wurmbii). MSc Thesis, University of Zurich
Clay Z, Furuichi T, de Waal FBM (2016) Obstacles and catalysts to peaceful coexistence in chimpanzees and bonobos. Behaviour 153:1293–1330. https://doi.org/10.1163/1568539X-00003335
Clutton-Brock TH, Parker GA (1992) Potential reproductive rates and the operation of sexual selection. Q Rev Biol 67:437–456
Darwin C (1871) The descent of man, and selection in relation to sex. Murray, London
Delgado RA, van Schaik CP (2000) The behavioral ecology and conservation of the orangutan (Pongo pygmaeus): a tale of two islands. Evol Anthropol 9:201–218. https://doi.org/10.1002/1520-6505(2000)9:5<201::AID-EVAN2>3.0.CO;2-Y
Deschner T, Boesch C (2007) Can the patterns of sexual swelling cycles in female Taï chimpanzees be explained by the cost-of-sexual-attraction hypothesis? Int J Primatol 28:389–406. https://doi.org/10.1007/s10764-007-9120-1
Deschner T, Heistermann M, Hodges K, Boesch C (2004) Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm Behav 46:204–215. https://doi.org/10.1016/j.yhbeh.2004.03.013
Douglas PH, Hohmann G, Murtagh R, Thiessen-Bock R, Deschner T (2016) Mixed messages: wild female bonobos show high variability in the timing of ovulation in relation to sexual swelling patterns. BMC Evol Biol 16:140. https://doi.org/10.1186/s12862-016-0691-3
Drea CM (2015) D’scent of man: a comparative survey of primate chemosignaling in relation to sex. Horm Behav 68:117–133. https://doi.org/10.1016/j.yhbeh.2014.08.001
Dunkel LP, Arora N, van Noordwijk MA, Utami Atmoko SS, Putra AP, Krützen M, van Schaik CP (2013) Variation in developmental arrest among male orangutans: a comparison between a Sumatran and a Bornean population. Front Zool 10:12. https://doi.org/10.1186/1742-9994-10-12
Emery Thompson M (2005) Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am J Primatol 67:137–158. https://doi.org/10.1002/ajp.20174
Emery Thompson M, Georgiev AV (2014) The high price of success: costs of mating effort in male primates. Int J Primatol 35:609–627. https://doi.org/10.1007/s10764-014-9790-4
Emery Thompson M, Kahlenberg SM, Gilby IC, Wrangham RW (2007) Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Anim Behav 73:501–512. https://doi.org/10.1016/j.anbehav.2006.09.007
Emery Thompson M, Knott CD (2008) Urinary C-peptide of insulin as a non-invasive marker of energy balance in wild orangutans. Horm Behav 53:526–535. https://doi.org/10.1016/j.yhbeh.2007.12.005
Emery Thompson M, Muller MN, Kahlenberg SM, Wrangham RW (2010) Dynamics of social and energetic stress in wild female chimpanzees. Horm Behav 58:440–449. https://doi.org/10.1016/j.yhbeh.2010.05.009
Emery Thompson M, Muller MN, Wrangham RW (2014) Male chimpanzees compromise the foraging success of their mates in Kibale National Park, Uganda. Behav Ecol Sociobiol 68:1973–1983. https://doi.org/10.1007/s00265-014-1803-y
Emery Thompson M, Stumpf RM, Pusey AE (2008) Female reproductive strategies and competition in apes: an introduction. Int J Primatol 29:815–821. https://doi.org/10.1007/s10764-008-9273-6
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Fox EA (1998) The function of female mate choice in Sumatran orangutans (Pongo abelii). PhD thesis, Duke University
Fox EA (2002) Female tactics to reduce sexual harassment in the Sumatran orangutan (Pongo pygmaeus abelii). Behav Ecol Sociobiol 52:93–101. https://doi.org/10.1007/s00265-002-0495-x
Fox J, Weisberg S (2018) An R companion to applied regression. Sage Publications, Thousand Oakes, CA
Furuichi T (2011) Female contributions to the peaceful nature of bonobo society. Evol Anthropol 20:131–142. https://doi.org/10.1002/evan.20308
Galdikas BMF (1981) Orangutan reproduction in the wild. In: Graham (ed) Reproductive biology of the great apes: comparative and biomedical perspectives. Academic Press, London, pp 281–300
Galdikas BMF (1985a) Adult male sociality and reproductive tactics among orangutans at Tanjung Puting. Folia Primatol 45:9–24
Galdikas BMF (1985b) Subadult male orangutan sociality and reproductive behavior at Tanjung Puting. Am J Primatol 8:87–99
Galdikas BMF (1988) Orangutan diet, range, and activity at Tanjung Puting, Central Borneo. Int J Primatol 9:1–35
Ganswindt A, Palme R, Heistermann M, Borragan S, Hodges JK (2003) Non-invasive assessment of adrenocortical function in the male African elephant (Loxodonta africana) and its relation to musth. Gen Comp Endocr 134:156–166
Georgiev AV, Russell AF, Emery Thompson M, Otali E, Muller MN, Wrangham RW (2014) The foraging costs of mating effort in male chimpanzees (Pan troglodytes schweinfurthii). Int J Primatol 35:725–745. https://doi.org/10.1007/s10764-014-9788-y
Hardus ME, de Vries H, Dellatore DF, Lameira AR, Menken SBJ, Wich SA (2013) Socioecological correlates of inter-individual variation in orangutan diets at Ketambe, Sumatra. Behav Ecol Sociobiol 67:429–437
Harrison ME, Morrogh-Bernard HC, Chivers DJ (2010) Orangutan energetics and the influence of fruit availability in the nonmasting peat-swamp forest of Sabangau, Indonesian Borneo. Int J Primatol 31:585–607. https://doi.org/10.1007/s10764-010-9415-5
Heistermann M, Ademmer C, Kaumanns W (2004) Ovarian cycle and effect of social changes on adrenal and ovarian function in Pygathrix nemaeus. Int J Primatol 25:689–708
Heistermann M, Palme R, Ganswindt A (2006) Comparison of different enzymeimmunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am J Primatol 68:257–273
Hinde RA, Atkinson S (1970) Assessing the roles of social partners in maintaining mutual proximity, as exemplified by mother-infant relations in rhesus monkeys. Anim Behav 18:169–176
Hodges KJ, Heistermann M (2011) Field endocrinology: monitoring hormonal changes in free-ranging primates. In: Setchell JM, Currtis DJ (eds) Field and laboratory methods in primatology: a practical guide. Cambridge University Press, Cambridge, pp 353–370
Hohmann G, Fruth B (2003) Intra- and inter-sexual aggression by bonobos in the context of mating. Behaviour 140:1389–1413
Hohmann G, Vigilant L, Mundry R, Behringer V, Surbeck M (2019) Aggression by male bonobos against immature individuals does not fit with predictions of infanticide. Aggressive Behav 45:300–309. https://doi.org/10.1002/ab.21819
Hrdy SB (1979) Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol 1:13–40
Hrdy SB, Whitten PL (1987) Patterning of sexual behavior. In: Smuts BB, Cheney DL, Seyfarth RM, Wranghym RW, Struhsaker TT (eds) Primate Societies. University of Chicago Press, Chicago, pp 370–384
Knott CD (2009) Orangutans : sexual coercion without sexual violence. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates and humans. Harvard University Press, Cambridge, pp 81–111
Knott CD, Beaudrot LH, Snaith T, White S, Tschauner H, Planansky G (2008) Female-female competition in Bornean orangutans. Int J Primatol 29:975–997. https://doi.org/10.1007/s10764-008-9278-1
Knott CD, Emery Thompson M, Stumpf RM, McIntyre MH (2010) Female reproductive strategies in orangutans, evidence for female choice and counterstrategies to infanticide in a species with frequent sexual coercion. Proc R Soc Lond B 277:105–113. https://doi.org/10.1098/rspb.2009.1552
Knott CD, Emery Thompson M, Wich SA (2009) The ecology of female reproduction in wild orangutans. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York, pp 171–188
Knott CD, Kahlenberg S (2007) Orangutans in perspective: forced copulations and female mating resistance. In: Bearder S, Campbell CJ, Fuentes A, MacKinnon KC, Panger M (eds) Primates in perspective. Oxford University Press, Oxford, pp 290–305
Knott CD, Scott AM, DiGiorgio A, Kane EE, Susanto TW, Riyandi R (2018) Are female orangutans less efficient foragers because of the risk of sexual coercion? In: 27th International Primatological Society Congress, Nairobi, Kenya (abstract)
Knott CD, Scott AM, O’Connell CA, Scott KS, Laman TG, Susanto TW (2019) Possible male infanticide in wild orangutans and a re-evaluation of infanticide risk. Sci Rep 9:7806. https://doi.org/10.1038/s41598-019-42856-w
Kunz JA (2020) Sexual conflict in orang-utans. PhD thesis, University of Zurich
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26
MacKinnon J (1974) The behaviour and ecology of wild orang-utans (Pongo pygmaeus). Anim Behav 22:3–74
Marty PR, van Noordwijk MA, Heistermann M, Willems EP, Dunkel LP, Cadilek M, Agil M, Weingrill T (2015) Endocrinological correlates of male bimaturism in wild Bornean orangutans. Am J Primatol 77:1170–1178. https://doi.org/10.1002/ajp.22453
Marzec AM, Kunz JA, Falkner S, Utami Atmoko SS, Alavi SE, Moldawer AM, Vogel ER, Schuppli C, van Schaik CP, van Noordwijk MA (2016) The dark side of the red ape: male-mediated lethal female competition in Bornean orangutans. Behav Ecol Sociobiol 70:459–466. https://doi.org/10.1007/s00265-015-2053-3
Matsumoto-Oda A (1998) Injuries to the sexual skin of female chimpanzees at Mahale and their effect on behaviour. Folia Primatol 69:400–404
Matsumoto-Oda A, Oda R, Hayashi Y, Murakami H, Maeda N, Kumazaki K, Shimizu K, Matsuzawa T (2003) Vaginal fatty acids produced by chimpanzees during menstrual cycles. Folia Primatol 74:75–79
Mesnick SL (1997) Sexual alliances: evidence and evolutionary implications. In: Gowaty PA (ed) Feminism and evolutionary biology: boundaries, intersections, and frontiers. Springer US, Boston, pp 207–260
Mitani JC (1985) Mating behaviour of male orangutans in the Kutai Game Reserve, Indonesia. Anim Behav 33:392–402
Mitani JC (1989) Orangutan activity budgets: monthly variations and the effects of body size, parturition, and sociality. Am J Primatol 18:87–100
Mitra Setia T, Delgado RA, Utami Atmoko SS, Singleton I, van Schaik CP (2009) Social organization and male-female relationships. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York, pp 245–253
Mitra Setia T, van Schaik CP (2007) The response of adult orang-utans to flanged male long calls: inferences about their function. Folia Primatol 78:215–226. https://doi.org/10.1159/000102317
Morrogh-Bernard HC, Husson SJ, Knott CD et al (2009) Orangutan activity budgets and diet. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York, pp 119–134
Muller MN, Emery Thompson M, Kahlenberg SM, Wrangham RW (2011) Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behav Ecol Sociobiol 65:921–933. https://doi.org/10.1007/s00265-010-1093-y
Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW (2007) Male coercion and the costs of promiscuous mating for female chimpanzees. Proc R Soc Lond B 274:1009–1014. https://doi.org/10.1098/rspb.2006.0206
Muller MN, Kahlenberg SM, Wrangham RW (2009) Male aggression and sexual coercion of females in primates. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates and humans. Harvard University Press, Cambridge, pp 3–22
Nadler RD (1981) Laboratory research on sexual behaviour of the great apes. In: Graham C (ed) Reproductive biology of the great apes: comparative and biomedical perspectives. Academic Press, New York, pp 191–238
Nieuwenhuis R, te Grotenhuis M, Pelzer B (2012) influence.ME: tools for detecting influential data in mixed effects models. R J 4:38–47
Nugraha TP, Heistermann M, Agil M, Purwantara B, Supriatna I, Gholib G, van Schaik CP, Weingrill T (2016) Validation of a field-friendly extraction and storage method to monitor fecal steroid metabolites in wild orangutans. Primates 58:285–294. https://doi.org/10.1007/s10329-016-0583-6
Nunn CL (1999) The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav 58:229–246. https://doi.org/10.1006/anbe.1999.1159
Nurmi NO, Hohmann G, Goldstone LG, Deschner T (2018) The “tolerant chimpanzee”— towards the costs and benefits of sociality in female bonobos. Behav Ecol 118:1–15. https://doi.org/10.1093/beheco/ary118
Paoli T (2009) The absence of sexual coercion in bonobos. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates and humans. Harvard University Press, Cambridge, pp 410–423
Parker GA (1979) Sexual selection and sexual conflict. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insects. Academic Press, London, pp 123–166
Pradhan GR, van Noordwijk MA, van Schaik CP (2012) A model for the evolution of developmental arrest in male orangutans. Am J Phys Anthropol 149:18–25. https://doi.org/10.1002/ajpa.22079
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Riedel J, Franz M, Boesch C (2011) How feeding competition determines female chimpanzee gregariousness and ranging in the Taï National Park, Côte d’Ivoire. Am J Primatol 73:305–313
Rijksen HD (1978) A field study on Sumatran orangutans (Pongo pygmaeus abelii): ecology, behavior and conservation. H.Veenman and Zonen, Wageningen
Roth TS, Rianti P, Fredriksson GM, Wich SA, Nowak MG (2020) Grouping behavior of Sumatran orangutans (Pongo abelii) and Tapanuli orangutans (Pongo tapanuliensis) living in forest with low fruit abundance. Am J Primatol 82:e23123. https://doi.org/10.1002/ajp.23123
Schultz AH (1938) Genital swelling in the female orang-utan. J Mammal 19:363–366
Schürmann CL, van Hooff JARAM (1986) Reproductive strategies of the orang-utan: new data and a reconsideration of existing sociosexual models. Int J Primatol 7:265–287
Scott AM, Knott CD, Susanto TW (2019) Are male orangutans a threat to infants? Evidence of mother–offspring counterstrategies to infanticide in Bornean orangutans (Pongo pygmaeus wurmbii). Int J Primatol 40:435–455
Silk J, Cheney D, Seyfarth R (2013) A practical guide to the study of social relationships. Evol Anthropol 22:213–225. https://doi.org/10.1002/evan.21367
Slater KY, Schaffner CM, Aureli F (2008) Female-directed male aggression in wild Ateles geoffroyi yucatanensis. Int J Primatol 29:1657–1669. https://doi.org/10.1007/s10764-008-9311-4
Smuts BB, Smuts RW (1993) Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv Stud Behav 22:1–63
Spillmann B, Dunkel LP, van Noordwijk MA, Amda RNA, Lameira AR, Wich SA, van Schaik CP (2010) Acoustic properties of long calls given by flanged male orang-utans (Pongo pygmaeus wurmbii) reflect both individual identity and context. Ethology 116:385–395. https://doi.org/10.1111/j.1439-0310.2010.01744.x
Spillmann B, Willems EP, van Noordwijk MA, Mitra Setia T, van Schaik CP (2017) Confrontational assessment in the roving male promiscuity mating system of the Bornean orangutan. Behav Ecol Sociobiol 71:20. https://doi.org/10.1007/s00265-016-2252-6
Stumpf RM, Boesch C (2010) Male aggression and sexual coercion in wild West African chimpanzees, Pan troglodytes verus. Anim Behav 79:333–342. https://doi.org/10.1016/j.anbehav.2009.11.008
Stumpf RM, Emery Thompson M, Knott CD (2008) A comparison of female mating strategies in Pan troglodytes and Pongo spp. Int J Primatol 29:865–884. https://doi.org/10.1007/s10764-008-9284-3
Sugardjito J, te Boekhorst IJA, van Hooff JA (1987) Ecological constraints on the grouping of wild orang-utans (Pongo pygmaeus) in the Gunung Leuser National Park, Sumatra, Indonesia. Int J Primatol 8:17–41
Therneau T (2018) coxme: mixed effects Cox models, https://cran.r-project.org/package=coxme
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871–1971. Aldine, Chicago, pp 136–179
Utami Atmoko SS, Mitra Setia T, Goossens B, James SS, Knott CD, Morrogh-Bernard HC, van Schaik CP, van Noordwijk MA (2009) Orangutan mating behavior and strategies. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York, pp 235–244
Utami Atmoko SS, van Hooff JA (2004) Alternative male reproductive strategies: male bimaturism in orangutans. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, Cambridge, pp 196–207
Utami Atmoko SS, Wich SA, Sterck EHM, van Hooff JA (1997) Food competition between wild orangutans in large fig trees. Int J Primatol 18:909–927
van de Pol M, Wright J (2009) A simple method for distinguishing within-versus between-subject effects using mixed models. Anim Behav 77:753–758
van Noordwijk MA, Arora N, Willems EP, Dunkel LP, Amda RNA, Mardianah N, Ackermann C, Krützen M, van Schaik CP (2012) Female philopatry and its social benefits among Bornean orangutans. Behav Ecol Sociobiol 66:823–834. https://doi.org/10.1007/s00265-012-1330-7
van Noordwijk MA, Sauren SEB, Nuzuar AA, Morrogh-Bernard HC, Utami Atmoko SS, van Schaik CP (2009) Development of independence. In: Wich SA, Utami Atmoko SS, Mitra-Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York, pp 189–203
van Noordwijk MA, Utami Atmoko SS, Knott CD, Kuze N, Morrogh-Bernard HC, Oram F, Schuppli C, van Schaik CP, Willems EP (2018) The slow ape: high infant survival and long interbirth intervals in wild orangutans. J Hum Evol 125:38–49. https://doi.org/10.1016/j.jhevol.2018.09.004
van Noordwijk MA, van Schaik CP (2000) Reproductive patterns in eutherian mammals: adaptations against infanticide? In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 322–360
van Noordwijk MA, van Schaik CP (2009) Intersexual food transfer among orangutans: do females test males for coercive tendency? Behav Ecol Sociobiol 63:883–890. https://doi.org/10.1007/s00265-009-0728-3
van Noordwijk MA, Willems EP, Utami Atmoko SS, Kuzawa CW, van Schaik CP (2013) Multi-year lactation and its consequences in Bornean orangutans (Pongo pygmaeus wurmbii). Behav Ecol Sociobiol 67:805–814. https://doi.org/10.1007/s00265-013-1504-y
van Schaik CP (2016) The primate origins of human nature. Wiley, Hoboken
van Schaik CP (1999) The socioecology of fission-fusion sociality in orangutans. Primates 40:69–86. https://doi.org/10.1007/BF02557703
van Schaik CP, Fox EA (1996) Temporal variability on orangutan gregariousness revisited. In: Proceedings from the Sixteenth Congress of the International Primatological Society held in Madison, Wisconsin (abstract)
van Schaik CP, Hodges JK, Nunn CL (2000) Paternity confusion and the ovarian cycles of female primates. In: van Schaik CP, Janson CH (eds) Infanticide by males its implications. Cambridge University Press, Cambridge, pp 361–387
van Schaik CP, Janson CH (eds) (2000) Infanticide by males and its implications. Cambridge University Press, Cambridge
van Schaik CP, Pradhan GR, van Noordwijk MA (2004) Mating conflict in primates: infanticide, sexual harassment and female sexuality. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, Cambridge, pp 141–163
van Schaik CP, van Hooff J (1983) On the ultimate causes of primate social systems. Behaviour 85:91–117
van Schaik CP, van Noordwijk MA, Vogel ER (2009) Ecological sex differences in wild orangutans. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York, pp 255–268
van Schaik CP, van Noordwijk MA, Wich SA (2006) Innovation in wild Bornean orangutans (Pongo pygmaeus wurmbii). Behaviour 143:839–876. https://doi.org/10.1163/156853906778017944
Vogel ER, Alavi SE, Utami Atmoko SS, van Noordwijk MA, Bransford TD, Erb WM, Zulfa A, Sulistyo F, Farida WR, Rothman JM (2017) Nutritional ecology of wild Bornean orangutans (Pongo pygmaeus wurmbii) in a peat swamp habitat: effects of age, sex, and season. Am J Primatol 79:1–20. https://doi.org/10.1002/ajp.22618
Vogel ER, Harrison ME, Zulfa A, Bransford TD, Alavi SE, Husson S, Morrogh-Bernard HC, Firtsman T, Utami Atmoko SS, van Noordwijk MA (2015) Nutritional differences between two orangutan habitats: implications for population density. PLoS One 10:e0138612. https://doi.org/10.5061/dryad.sk616
Wartmann FM, Purves RS, van Schaik CP (2010) Modelling ranging behaviour of female orang-utans: a case study in Tuanan, Central Kalimantan, Indonesia. Primates 51:119–130. https://doi.org/10.1007/s10329-009-0186-6
Weingrill T, Willems EP, Zimmermann N, Steinmetz H, Heistermann M (2011) Species-specific patterns in fecal glucocorticoid and androgen levels in zoo-living orangutans (Pongo spp.). Gen Comp Endocr 172:446–457. https://doi.org/10.1016/j.ygcen.2011.04.008
Wich SA, Geurts ML, Mitra Setia T (2006) Influence of fruit availability on Sumatran orangutan sociality and reproduction. In: Hohmann G, Robbins MM, Boesch C (eds) Feeding ecology in apes and other primates. Ecological, physical and behavioral aspects. Cambridge University Press, New York, pp 335–356
Wich SA, Vogel ER, Larsen MD, Fredriksson G, Leighton M, Yeager CP, Brearley FQ, van Schaik CP, Marshall AJ (2011) Forest fruit production is higher on Sumatra than on Borneo. PLoS One 6:e21278. https://doi.org/10.1371/journal.pone.0021278
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wilke CO (2019) cowplot: streamlined plot theme and plot annotations for “ggplot2.” http://bioconductor.statistik.tu-dortmund.de/cran/web/packages/cowplot/cowplot.pdf
Wilson ML, Boesch C, Fruth B, Furuichi T, Gilby IC, Hashimoto C, Hobaiter CL, Hohmann G, Itoh N, Koops K, Lloyd JN, Matsuzawa T, Mitani JC, Mjungu DC, Morgan D, Muller MN, Mundry R, Nakamura M, Pruetz J, Pusey AE, Riedel J, Sanz C, Schel AM, Simmons N, Waller M, Watts DP, White F, Wittig RM, Zuberbühler K, Wrangham RW (2014) Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513:414–417. https://doi.org/10.1038/nature13727
Wrangham RW (2002) The cost of sexual attraction: is there a trade-off in female Pan between sex appeal and received coercion? In: Boesch C, Hohmann G, Marchand LF (eds) Behavioural diversity in chimpanzees and bonobos. Cambridge University Press, Cambridge, pp 204–215
Zinner DP, Nunn CL, van Schaik CP, Kappeler PM (2004) Sexual selection and exaggerated sexual swellings of female primates. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, New York, pp 71–89
Acknowledgments
We thank our local field teams at Suaq and Tuanan and all the local and foreign students and researchers for their contribution in the long-term data collection. Particularly, we express our gratitude to Dr. Alison Ashbury, Rebecca Brittain, Manon Bodin, Lynda P. Dunkel, Caroline Fryns, Dr. Anna Marzec, Dr. Caroline Schuppli, Dr. Brigitte Spillmann, and Sofia Vileila. We thank Universitas Nasional (UNAS) for support and collaboration and particularly Dr. Tatang Mitra Setia, Astri Zulfa, Misdi bin Abdullah, and Kristana P. Makur. We thank the Indonesian State Ministry for Research and Technology (RISTEK), the Indonesian Institute of Sciences (LIPI), the Ministry of Environment and Forestry (KLHK), the Ministry of Internal Affairs, Indonesia, the local government in Central Kalimantan, the BKSDA Palangkaraya, the Bornean Orangutan Survival Foundation (BOSF), MAWAS in Palangkaraya, the Sumatran orangutan conservation Program (SOCP), and Balai Besar Taman Nasional Gunung Leuser (BBTNGL) in Medan for their permission and support to conduct this study. We are grateful to Andrea Heistermann, Dr. Gholib, and Joshua Reukauf for their support during fecal sample analyses. Fecal sample collection was conducted according to Indonesian and international regulations and with the kind permission of the Indonesian Ministry of Environment and Forestry (KLHK) (permit numbers: 17256/IV/SA TS-LN/2012, SK.49/KSDAE/SET/KSA.2/1/2017). We thank Kevin Langergraber and two anonymous reviewers for helpful comments.
Funding
Open Access funding provided by University of Zurich. This work was supported by the University of Zurich, the A. H. Schultz Foundation, Janggen Pöhn Foundation and the Swiss National Science Foundation (grant no. 310030B_160363/1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is an observational study and fecal samples were collected non-invasively. Observers did not interact with the wild focal individuals in any way and kept a minimum distance of 10 m in order to minimize any effect on their natural behavior. The data collection protocol for this study adheres to legal requirements of Indonesia and was approved by the Indonesian State Ministry for Research and Technology (RISTEK) (permit number 66/SIP/FRP/E5/Dit.KI/III/2016), the Directorate General of Natural Resources and Ecosystem Conservation- Ministry of Environment and Forestry of Indonesia (KSDAE-KLHK), the Ministry of Internal Affairs, Indonesia, the Nature Conservation Agency of Central Kalimantan (BKSDA) and Balai Besar Taman Nasional Gunung Leuser (BBTNGL). Fecal sample collection was conducted according to Indonesian and international regulations and with the permission of the Indonesian Ministry of Environment and Forestry (KLHK) (permit numbers: 17256/IV/SA TS-LN/2012, SK.49/KSDAE/SET/KSA.2/1/2017).
Informed consent
Not applicable
Additional information
Communicated by K. Langergraber
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1.09 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kunz, J.A., Duvot, G.J., van Noordwijk, M.A. et al. The cost of associating with males for Bornean and Sumatran female orangutans: a hidden form of sexual conflict?. Behav Ecol Sociobiol 75, 6 (2021). https://doi.org/10.1007/s00265-020-02948-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02948-4