Abstract
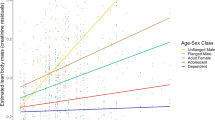
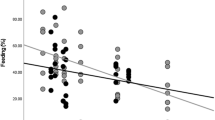
Data on energy intake and the effects of fluctuations in fruit availability on energy intake for African apes, and orangutans in mast-fruiting habitats, indicate that orangutans may face greater energetic challenges than do their African counterparts. Comparable data on orangutans in nonmasting forests, which experience lower fluctuations in fruit availability, have been lacking, however, complicating interpretations. We conducted a 46-mo study of orangutan energetics in the nonmasting Sabangau peat-swamp forest, Indonesian Borneo. Sabangau orangutans experienced periods of negative energy balance apparently even longer than in mast-fruiting habitats, as indicated by comparisons of observed energy intake with theoretical requirements and analysis of urinary ketones. Daily energy intake was positively related to fruit availability in flanged males, but not in adult females or unflanged males. This may represent different foraging strategies between age-sex classes and suggests that fruit availability is not always an accurate indicator of ape energy intake/balance. Urinary ketone levels were not generally related to fruit availability, daily energy intake, day range, or party size. This is probably due to low energy intake, and consequently high ketone production, throughout much of the study period. Comparisons with published results on African apes support the hypothesis that orangutans are unique among hominoids in regularly experiencing prolonged periods of negative energy balance. This has important effects on orangutan behavior and socioecology, and has likely been a key factor driving the evolutionary divergence of orangutans and African apes.



Similar content being viewed by others
References
Abbott, W. G. H., Howard, B. V., Christin, L., Freymond, D., Lillioja, S., Boyce, V. L., et al. (1988). Short-term energy balance: relationship with protein, carbohydrate, and fat balances. American Journal of Physiology, 255, E332–E337.
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49, 227–265.
Altmann, J. (1983). Costs of reproduction in baboons (Papio cynocephalus). In W. P. Aspey & S. I. Lustik (Eds.), Behavioral energetics: The cost of survival in vertebrates (pp. 67–88). Columbus: Ohio State University Press.
Altmann, J., & Samuels, A. (1992). Costs of maternal care: infant-carrying in baboons. Behavioral Ecology and Sociobiology, 29, 391–398.
Andrews, P. (1996). Palaeoecology and hominoid palaeoenvironments. Biological Reviews of the Cambridge Philosophical Society, 71, 257–300.
Bharatu, S., Pal, M., Bhattacharya, B. N., & Bharati, P. (2007). Prevalence and causes of chronic energy deficiency and obesity in Indian women. Human Biology, 79, 395–412.
Blanc, S., Scheller, D., Kemnitz, J., Weindruch, R., Colman, R., Newton, W., et al. (2003). Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. The Journal of Clinical Endocrinology and Metabolism, 88, 16–23.
BSN. (1992). Cara Uji Makanan dan Minuman [Methods for testing foods and drinks]. Jakarta: Badan Standardisasi Nasional.
Cannon, C. H., Curran, L. M., Marshall, A. J., & Leighton, M. (2007). Long-term reproductive behaviour of woody plants across seven Bornean forest types in the Gunung Palung National Park (Indonesia): suprannual synchrony, temporal productivity and fruiting diversity. Ecological Letters, 10, 956–969.
Chapman, C. A., Chapman, L. J., Rode, K. D., Hauck, E. M., & McDowell, L. R. (2003). Variation in the nutritional value of primate foods: Among trees, time periods, and areas. International Journal of Primatology, 24, 317–333.
Cocks, L. (2007). Factors influencing the well-being and longevity of captive female orangutans. International Journal of Primatology, 28, 429–440. doi:10.1007/s10764-007-9117-9.
Coehlo, A. M. (1986). Time and energy budgets. In A. R. Liss (Ed.), Comparative primate biology (pp. 141–66). New York: Alan R. Liss.
Colman, R. J., Anderson, R. M., Johnson, S. C., Kastman, E. K., Kosmatka, K. J., Beasley, T. M., et al. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science, 325, 201–204.
Conklin, N. L., & Wrangham, R. W. (1994). The value of figs to a hind-gut fermenting frugivore: a nutritional analysis. Biochemical Systematics and Ecology, 22, 137–151.
Conklin-Brittain, N. L., Knott, C. D., & Wrangham, R. W. (2006). Energy intake by wild chimpanzees and orangutans: Methodological considerations and a preliminary comparison. In G. Hohmann, M. M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates. Ecological, physical and behavioral aspects (pp. 445–471). Cambridge: Cambridge University Press.
de Bonis, L., & Koufos, G. D. (1993). The face and the mandible of Ouranopithecus macedoniensis: description of new specimens and comparisons. Journal of Human Evolution, 24, 469–491.
Deschner, T., Kratzsch, J., & Hohmann, G. (2008). Urinary C-peptide as a method for monitoring body mass changes in captive bonobos (Pan paniscus). Hormones and Behavior, 54, 620-626. doi:10.1016/j.yhbeh.2008.06.005.
Durnin, J. V. G. A. (1979). Energy balance in man with particular reference to low energy intakes. Nutritio et Dieta, 27, 1–10.
Durnin, J. V. G. A., Edholm, O. G., Miller, D. S., & Waterlow, J. C. (1973). How much food does man require? Nature, 242, 418.
Edmundson, W. (1980). Adaptation to undernutrition: how much food does man need? Social Science & Medicine, 14D, 119–126.
Emery Thompson, M., & Knott, C. D. (2008). Urinary C-peptide of insulin as a non-invasive marker of energy balance in wild orangutans. Hormones and Behavior, 53, 526–535.
Garrow, J. S., & Webster, J. D. (1984). Thermogenesis to small stimuli. In A. J. M. Van Ess (Ed.), Human energy metabolism (pp. 215–224). Wageningen: European Community Concerted Action on Nutrition and Health (EURONUT) Report 5.
Goering, H. K., & van Soest, P. J. (1970). Forage fiber analysis. In H. K. Goering (Ed.), Agricultural Handbook. Number 379. Washington, DC: United States Department of Agriculture, Agricultural Research Service.
Hannibal, D. L., & Guatelli-Steinberg, D. (2005). Linear enamel hypoplasia in the great apes: analysis by genus and locality. American Journal of Physical Anthropology, 127, 13–25.
Harrison, M. E. (2009). Orang-utan feeding behaviour in Sabangau, Central Kalimantan. PhD thesis, University of Cambridge, Cambridge.
Harrison, M. E., & Chivers, D. J. (2007). The orang-utan mating system and the unflanged male: a product of declining food availability during the late Miocene and Pliocene? Journal of Human Evolution, 52, 275–293.
Harrison, M. E., Vogel, E. R., Morrogh-Bernard, H., & van Noordwijk, M. A. (2009). Methods for calculating activity budgets compared: a case study using orangutans. American Journal of Primatology, 71, 353–358.
Holloszya, J. O., & Fontana, L. (2007). Caloric restriction in humans. Experimental Gerontology, 42, 709–712.
Kelly, T. R., Sleeman, J. M., & Wrangham, R. W. (2004). Urinalysis in free-living chimpanzees (Pan troglodytes schweinfurthii) in Uganda. The Veterinary Record, 154, 729–730.
Key, C., & Ross, C. (1999). Sex differences in energy expenditure in non-human primates. Proceedings of the Royal Society of London. Series B, 266, 2479–2485.
Knott, C. D. (1998). Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. International Journal of Primatology, 19, 1061–1079.
Knott, C. D. (1999). Reproductive, physiological and behavioural responses of orangutans in Borneo to fluctuations in food availability. PhD thesis, Harvard University, Cambridge, MA.
Knott, C. D. (2001). Female reproductive ecology of the apes: Implications for human evolution. In P. T. Ellison (Ed.), Reproductive ecology and human evolution (pp. 429–463). New York: Walter de Gruyter.
Knott, C. D. (2005). Energetic responses to food availability in the great apes: Implications for hominin evolution. In D. Brockman & C. P. van Schaik (Eds.), Primate seasonality: Implications for human evolution (pp. 351–378). Cambridge: Cambridge University Press.
Kurpad, A. V., Muthayya, S., & Vaz, M. (2005). Consequences of inadequate food energy and negative energy balance in humans. Public Health Nutrition, 8, 1053–1076.
Leibel, R. L., Rosenbaum, M., & Hirsch, J. (1995). Changes in energy expenditure resulting from altered body weight. The New England Journal of Medicine, 332, 621–628.
Leighton, M. (1993). Modelling dietary selectivity by Bornean orangutans: evidence of multiple criteria in fruit selection. International Journal of Primatology, 14, 257–313.
Leonard, W. R., & Robertson, M. L. (1997). Comparative primate energetics and evolution. American Journal of Physical Anthropology, 102, 265–281.
Levine, J. A., Schleusner, S. J., & Jensen, M. D. (2000). Energy expenditure of nonexercise activity. The American Journal of Clinical Nutrition, 72, 1451–1454.
Manduell, K. (2008). Locomotor behaviour of wild orangutans (P. p. wurmbii) in disturbed peat swamp forest, Sabangau, Central Kalimantan, Indonesia. MRes thesis, Manchester Metropolitan University, Manchester, UK.
Markham, R., & Groves, C. P. (1990). Brief communication: weights of wild orang-utans. American Journal of Physical Anthropology, 81, 1–3.
Marshall, A. J., & Wrangham, R. W. (2007). Evolutionary consequences of fallback foods. International Journal of Primatology, 28, 1219–1235.
Marshall, A. J., Ancrenaz, M., Brearley, F. Q., Fredriksson, G. M., Ghaffar, N., Heydon, M., et al. (2009). The effects of forest phenology and floristics on populations of Bornean and Sumatran orangutans: Are Sumatran forests better orangutan habitat than Bornean forests? In S. A. Wich, S. S. Utami Atmoko, T. Mitra Setia, & C. P. van Schaik (Eds.), Orangutans: Geographic variation in behavioral ecology and conservation (pp. 97–116). Oxford: Oxford University Press.
Masi, S. (2008). Seasonal influence on foraging strategies, activity and energy budgets of western lowland gorillas (Gorilla gorilla gorilla) in Bai Hokou, Central African Republic. PhD thesis, University of Rome La Sapienza, Rome, Italy.
Milton, K., & Demment, M. W. (1988). Digestion and passage kinetics of chimpanzees fed high and low fibre diets and comparisons with human data. The Journal of Nutrition, 118, 1082–1088.
Morrogh-Bernard, H. (2009). Orang-utan behavioural ecology in the Sabangau Peat-Swamp Forest, Borneo. PhD thesis, University of Cambridge, Cambridge.
Morrogh-Bernard, H., Husson, S., & McLardy, C. (2002). Orang-utan data collection standardisation. Designed during Orang-utan Culture Workshop, February 2002, San Anselmo, CA.
Morrogh-Bernard, H., Husson, S., Page, S. E., & Rieley, J. O. (2003). Population status of the Bornean orang-utan (Pongo pygmaeus) in the Sebangau peat swamp forest, Central Kalimantan, Indonesia. Biological Conservation, 110, 141–152.
Morrogh-Bernard, H. C., Husson, S. J., Knott, C. D., Wich, S. A., van Schaik, C. P., van Noordwijk, M. A., et al. (2009). Orangutan activity budgets and diet: A comparison between species, populations and habitats. In S. A. Wich, S. S. Utami Atmoko, T. Mitra Setia, & C. P. van Schaik (Eds.), Orangutans: Geographic variation in behavioral ecology and conservation (pp. 119–133). Oxford: Oxford University Press.
NAS/National Academy of Sciences (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC, Food and Nutrition Board of the Institute of Medicine of the National Academies, National Academies Press.
Nieburg, P., Person-Karell, B., & Toole, M. J. (1992). Malnutrition-mortality relationships among refugees. Journal of Refugee Studies, 5, 247–256.
Nkurunungi, J. B., Ganas, J., Robbins, M. M., & Stanford, C. B. (2004). A comparison of two mountain gorilla habitats in Bwindi Impenetrable National Park, Uganda. African Journal of Ecology, 42, 289–297.
NRC. (2003). Nutrient requirements of nonhuman primates (2nd ed.). Washington: The National Research Council. The National Academies Press.
Oyarzun, S. E., Crawshaw, G. J., & Vaides, E. V. (1996). Nutrition of the tamandua: I. Nutrient composition of termites (Nasutitermes spp.) and stomach contents from wild tamanduas (Tamandua tetradactyla). Zoo Biology, 15, 509–524.
Page, S. E., Rieley, J. O., Shotyk, Ø. W., & Weiss, D. (1999). Interdependence of peat and vegetation in a tropical peat swamp forest. Philosophical Transactions of the Royal Society of London. B, 354, 1885–1897.
Pierce, W. C., & Haenish, E. L. (1947). Quantitative analysis. London: Wiley.
Pontzer, H., & Wrangham, R. W. (2004). Climbing and the daily energy cost of locomotion in wild chimpanzees: implications for hominoid locomotor evolution. Journal of Human Evolution, 46, 317–335.
Potts, K. B. (2008). Habitat heterogeneity on multiple spatial scales in Kibale National Park, Uganda: Implications for chimpanzee population ecology and grouping patterns. PhD thesis, Yale University, New Haven, CT.
Remis, M. J. (2002). Food preferences among captive Western gorillas (Gorilla gorilla gorilla) and chimpanzees (Pan troglodytes). International Journal of Primatology, 23, 231–249.
Remis, M. J. (2003). Are gorillas vacuum cleaners of the forest floor? The roles of body size, habitat, and food preferences on dietary flexibility and nutrition. In A. B. Taylor & M. L. Goldsmith (Eds.), Gorilla biology: A multidisciplinary perspective (pp. 385–404). Cambridge: Cambridge University Press.
Robertson, J. B., & van Soest, P. J. (1980). The detergent system of analysis and its application to human foods. In W. P. T. James & O. Theander (Eds.), The analysis of dietary fiber in food (pp. 123–158). New York: Marcel Decker.
Robinson, A. M. (1980). Physiological role of ketone bodies as substrates and signals in mammalian tissues. Physiological Reviews, 60, 143.
Rothman, J. M., Dierenfeld, E. S., Hintz, H. F., & Pell, A. N. (2008). Nutritional quality of gorilla diets: Consequences of age, sex, and season. Oecologia, 155, 111–122.
Schmidt, D. A., Kerley, M. S., Dempsey, J. L., Porton, I. J., Porter, J. H., Griffin, M. E., et al. (2005). Fiber digestibility by the orangutan (Pongo abelii) in vitro and in vivo. Journal of Zoo and Wildlife Medicine, 36, 571–580.
Schofield, S., & Lambert, C. M. (1975). Village nutrition studies: An annotated bibliography. University of Sussex: Institute of Development Studies.
Scott, M. L. (1986). Energy requirements, sources, and metabolism. In: Nutrition in humans and selected animal species (pp. 12–78). New York: Wiley.
Sherry, D. S., & Ellison, P. T. (2007). Potential applications of urinary C-peptide of insulin for comparative energetics research. American Journal of Physical Anthropology, 133, 771–778. doi:10.1002/ajpa.20562.
Shetty, P. S. (1993). Chronic undernutrition and metabolic adaptation. The Proceedings of the Nutrition Society, 52, 267–284.
Shetty, P. (2005). Energy requirements of adults. Public Health Nutrition, 8, 994–1009. doi:10.1079/PHN2005792.
Thorpe, S. K. S., & Crompton, R. H. (2009). Orangutan positional behavior: Interspecific variation and ecological correlates. In S. A. Wich, S. S. Utami Atmoko, T. Mitra Setia, & C. P. van Schaik (Eds.), Orangutans: Geographic variation in behavioral ecology and conservation (pp. 33–47). Oxford: Oxford University Press.
Thorpe, S. K. S., Crompton, R. H. & Alexander, R. M. (2007). Orangutans use compliant branches to lower the energetic cost of locomotion. Biology Letters, 3, 253-256. doi:10.1098/rsbl.2007.0049.
van Schaik, C. P. (1996). Strangling figs: Their role in the forest. In C. P. van Schaik & J. Supriatna (Eds.), Leuser: A Sumatran sanctuary (pp. 111–119). Jakarta: Perdana Ciptamadri.
van Schaik, C. P. (1999). The socioecology of fission-fusion sociality in orangutans. Primates, 40, 69–86.
van Schaik, C. P., & Pfannes, K. R. (2005). Tropical climates and phenology: a primate perspective. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in primates: Studies of living and extinct human and nonhuman primates (pp. 23–54). Cambridge: Cambridge University Press.
van Soest, P. J. (1994). Nutritional ecology of the ruminant (2nd ed.). Ithaca: Comstock Publishing Associates.
Vogel, E. R., van Woerden, J. T., Lucas, P. W., Utami Atmoko, S. S., van Schaik, C. P., & Dominy, N. J. (2008). Functional ecology and evolution of hominoid molar enamel thickness: Pan troglodytes schweinfurthii and Pongo pygmaeus wurmbii. Journal of Human Evolution, 55, 60–74.
Waterlow, J. C. (1986). Metabolic adaptation to low intakes of energy and protein. Annual Review of Nutrition, 6, 495–526.
WFP. (2007). World Hunger Series 2007: Hunger and health. Rome, Italy: World Food Programme.
Wheatley, B. P. (1982). Energetics of foraging in Macaca fascicularis and Pongo pygmaeus and a selective advantage of large body size in the orangutan. Primates, 23, 348–363.
Wheatley, B. P. (1987). The evolution of large body size in orangutans: a model for hominoid divergence. American Journal of Primatology, 13, 313–324.
Wich, S. A., Geurts, M. L., Mitra Setia, T., & Utami-Atmoko, S. S. (2006). Influence of fruit availability on Sumatran orangutan sociality and reproduction. In G. Hohmann, M. M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates. Ecological, physical and behavioral aspects (pp. 335–356). Cambridge: Cambridge University Press.
Wich, S. A., Utami-Atmoko, S. S., Mitra Setia, T., Djojosudharmo, S., & Geurts, M. L. (2006). Dietary and energetic responses of Pongo abelii to fruit availability fluctuations. International Journal of Primatology, 27, 1535–1550.
Wich, S. A., Meijaard, E., Marshall, A. J., Husson, S., Ancrenaz, M., Lacy, R. C., et al. (2008). Distribution and conservation status of the orangutan (Pongo spp.) on Borneo and Sumatra: how many remain? Oryx, 42, 329–339.
Wrangham, R. W., Conklin-Brittain, N. L., & Hunt, K. D. (1998). Dietary responses of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants. International Journal of Primatology, 19, 949–970.
Young, H., & Jaspars, S. (1995). Nutrition, disease and death in times of famine. Disasters, 19, 94–109.
Acknowledgments
We thank the Indonesian Institute of Sciences (LIPI), Director General of Nature Conservation (PHKA), and Centre for the International Cooperation in Management of Tropical Peatlands (CIMTROP) for research permissions. Funding was provided by the Leakey Foundation, Wildlife Conservation Society, U.S. Fish and Wildlife Service, Wingate Foundation, Primate Conservation Inc., Conservation International Primate Action Fund, IdeaWild, Cambridge Philosophical Society, Columbus Zoo, the Rufford Foundation, and the Orangutan Tropical Peatland Project (OuTrop). Simon Husson, Susan Cheyne, and Suwido Limin provided support and assistance throughout. We thank Cheryl Knott, Susan Cheyne, Nancy Conklin-Brittain, Kevin Potts, Ben Buckley, and Kirsten Manduell for information and comments that helped improve the manuscript. We especially thank the numerous field assistants and students who helped with data collection, and W. Rosa Farida and Tri Hadi Handayani for performing nutritional analyses. The editor and 2 anonymous reviewers provided comments that improved the manuscript. This paper is dedicated in loving memory of Jery Yenyahu Akar (“Zeri”): fantastic assistant, true anak rimba, and friend.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harrison, M.E., Morrogh-Bernard, H.C. & Chivers, D.J. Orangutan Energetics and the Influence of Fruit Availability in the Nonmasting Peat-swamp Forest of Sabangau, Indonesian Borneo. Int J Primatol 31, 585–607 (2010). https://doi.org/10.1007/s10764-010-9415-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9415-5