Abstract
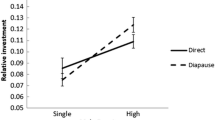
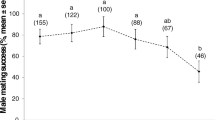
Contrary to vertebrates, sperm production in insects may bear considerable costs for males. This is especially true in species that donate spermatophores containing sperm and nutrient-rich accessory gland products like in butterflies. Hence, spermatophores at first and subsequent copulations can differ in a quantitative and qualitative way. Such effects have particularly been shown in polyandrous species providing large spermatophores. Here we experimentally tested the effect of male mating status (virgin male vs recently mated male) on copulation duration, spermatophore size and females’ fitness components in a monandrous butterfly Pararge aegeria that typically donates small spermatophores. Copulations with non-virgin males lasted on average five times longer than that with virgin males and resulted in a spermatophore which was on average three times smaller. Number of eggs laid and female life span were not affected by the mating status treatment, but there was a significant effect on the number of living caterpillars a female produced, as copulations with virgin males resulted in higher numbers of larval offspring. Interestingly, the difference in spermatophore mass at the first and the second copulation increased with male body size. This suggests differential spermatophore allocation decisions among males of different size. Consequences for females and potential mechanisms influencing female fitness components are discussed. Given the small absolute size of spermatophores in P. aegeria, components other than consumable nutrients (perhaps hormones) should cause the observed effects.



Similar content being viewed by others
References
Bissoondath CJ, Wiklund C (1995) Protein content of spermatophores in relation to monandry/polyandry in butterflies. Behav Ecol Sociobiol 37:365–371
Bissoondath CJ, Wiklund C (1996a) Effect of male mating history and body size on ejaculate size and quality in two polyandrous butterflies Pieris napi and P. rapae (Lepidoptera: Pieridae). Funct Ecol 10:457–464
Bissoondath CJ, Wiklund C (1996b) Male butterfly investment in successive ejaculates in relation to mating system. Behav Ecol Sociobiol 39:285–292
Boggs CL (1990) A general model of the role of male-donated nutrients in female insects’ reproduction. Am Nat 136:598–617
Boggs CL (1997) Reproductive allocation from reserves and income in butterfly species with differing adult diets. Ecology 78:181–191
Boggs CL (2003) Environmental variation, life histories, and allocation. In: Boggs CL, Wat WB, Ehrlich PR (eds) Butterflies: ecology and evolution taking flight. University of Chicago Press, Chicago, pp 185–206
Boggs CL, Gilbert LE (1979) Male contribution to egg production in butterflies: evidence for transfer of nutrients at mating. Science 206:83–84
Dewsbury DA (1982) Ejaculate cost and male choice. Am Nat 119:601–610
Gillott C (2003) Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu Rev Entomol 48:163–184
Hack MA (1997) The effects of mass and age on standard metabolic rate in house crickets. Physiol Entomol 22:325–331
Hughes L, Chang BSW, Wagner D, Pierce NE (2000) Effects of mating history on ejaculate size, fecundity, longevity, and copulation duration in the ant-tended lycaenid butterfly, Jalmenus evagoras. Behav Ecol Sociobiol 47:119–128
Kaitala A, Wiklund C (1994) Polyandrous females forage for matings. Behav Ecol Sociobiol 35:385–388
Karlsson B (1987) Variation in egg weight, oviposition rate and reproductive reserves with female age in a natural population of the speckled wood butterfly, Pararge aegeria. Ecol Entomol 12:473–476
Karlsson B (1998) Nuptial gifts, resource budgets, and reproductive output in a polyandrous butterfly. Ecology 79:2931–2940
Karlsson B, Van Dyck H (2005) Does habitat fragmentation affect temperature-related life-history traits? A laboratory test with a woodland butterfly. Proc R Soc B 272:1257–1263
Karlsson B, Wickman PO (1990) Increased reproductive effort as explained by body size and resource allocation in the speckled wood butterfly, Pararge aegeria (L.). Funct Ecol 4:609–617
Karube F, Kobayashi M (1999) Presence of eupyrene spermatrozoa in vestibulum accelerates oviposition in the silkworm moth, Bombyx mori. J Insect Physiol 45:947–957
Krebs JR, Davies NB (1993) Behavioural ecology: an evolutionary approach. Cambridge University Press, Cambridge
Kubli E (2003) Sex-peptides: seminal peptides of the Drosophila male. Cell Mol Life Sci 60:1689–1704
Lange AB, Loughton BG (1985) An oviposition-stimulating factor in the male accessory reproductive gland of the locust, Locusta migratoria. Gen Comp Endocrinol 57:208–215
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute, Cary, NC
Molleman F, Zwaan BJ, Brakefield PM (2004) The effect of male sodium diet and mating history on female reproduction in the puddling squinting bush brown Bicyclus anynana (Lepidoptera). Behav Ecol Sociobiol 56:404–411
Nylin S, Wickman PO, Wiklund C (1995) Life-cycle regulation and life history plasticity in the speckled wood butterfly: are reaction norms predictable? Biol J Linn Soc 55:143–157
Oberhauser KS (1989) Effects of spermatophores on male and female monarch butterfly mating success. Behav Ecol Sociobiol 25:237–246
Pivnick KA, McNeil JN (1987) Puddling in butterflies: sodium affects reproductive success in Thymelicus lineola. Physiol Entomol 12:461–472
Royer L, McNeil JN (1993) Male investment in the European corn-borer, Ostrinia nubilalis (Lepidoptera, Pyralidae)—impact on female longevity and reproductive performance. Funct Ecol 7:209–215
Rutowski RL, Gilchrist GW (1986) Copulation in Colias eurytheme (Lepidoptera: Pieridae): patterns and frequency. J Zool (Lond) 209:115–124
Rutowski RL, Gilchrist GW, Terkanian B (1987) Female butterflies mated with recently mated males show reduced reproductive output. Behav Ecol Sociobiol 20:319–322
Shreeve TG (1986) Egg-laying by the speckled wood (Pararge aegeria): the role of female behaviour, host plant abundance and temperature. Ecol Entomol 11:229–236
Simmons LW (1993) Some constraints on reproduction for male bush-crickets, Requena verticalis (Orthoptera, Tettigoniidae)—diet, size and parasite load. Behav Ecol Sociobiol 32:135–139
Svärd L (1985) Paternal investment in a monandrous butterfly, Pararge aegeria. Oikos 45:66–70
Svärd L, Wiklund C (1986) Different ejaculate delivery strategies in first versus subsequent matings in the swallowtail butterfly Papilio machaon L. Behav Ecol Sociobiol 18:325–330
Svärd L, Wiklund C (1988) Fecundity, egg weight and longevity in relation to multiple matings in females of the monarch butterfly. Behav Ecol Sociobiol 23:39–43
Svärd L, Wiklund C (1989) Mass and production rate of ejaculates in relation to monandry/polyandry in butterflies in butterflies. Behav Ecol Sociobiol 24:395–402
Svärd L, Wiklund C (1991) The effect of ejaculate mass on female reproductive output in the European swallowtail butterfly, Papilio machaon (l) (Lepidoptera, Papilionidae). J Insect Behav 4:33–41
Tamhankar AJ (1995) Host influence on mating-behavior and spermatophore reception correlated with reproductive output and longevity of female Earias insulana (boisduval) (Lepidoptera, Noctuidae). J Insect Behav 8:499–511
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Cambridge
Tolman T, Lewington R (1997) Butterflies of Britain and Europe. Harper Collins, London
Trivers RL (1972) Paternal investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine, Chicago, pp 136–179
Torres-Vila LM, Jennions MD (2005) Male mating history and female fecundity in the Lepidoptera: do male virgins make better partners? Behav Ecol Sociobiol 57:318–326
Torres-Vila LM, Rodriguez-Molina MC (2002) Egg size variation and its relationship with larval performance in the Lepidoptera: the case of the European grapevine moth Lobesia botrana. Oikos 99:272–283
Torres-Vila LM, Rodriguez-Molina MC, Jennions MD (2004) Polyandry and fecundity in the Lepidoptera: can methodological and conceptual approaches bias outcomes? Behav Ecol Sociobiol 55:315–324
Van Dyck H (2003) Mate location, a matter of design? Adaptive morphological variation in the speckled wood butterfly. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies: evolution and ecology taking flight. University of Chicago Press, Chicago, pp 353–366
Ward KE, Landolt PJ (1995) Influence of multiple matings on fecundity and longevity of female cabbage-looper moths (Lepidoptera, Noctuidae). Ann Entomol Soc Am 88:768–772
Watanabe M, Wiklund C, Bon’no M (1998) The effect of repeated matings on sperm numbers in successive ejaculates of the cabbage white butterfly Pieris rapae. J Insect Behav 11:559–570
Wedell N (1993) Mating effort or paternal investment—incorporation rate and cost of male donations in the wartbiter. Behav Ecol Sociobiol 32:239–246
Wedell N (1996) Mate quality affects reproductive effort in a paternally investing species. Am Nat 48:1075–1088
Wedell N, Karlsson B (2003) Paternal investment directly affects female reproductive effort in an insect. Proc R Soc Lond B 270:2065–2071
Wickman PO, Karlsson B (1987) Changes in egg color, egg weight and oviposition rate with the number of eggs laid by wild females of the small heath butterfly, Coenonympha pamphilus. Ecol Entomol 12:109–114
Wiklund C (2003) Sexual selection and the evolution of butterfly mating systems. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies: ecology and evolution taking flight. University of Chicago Press, Chicago, pp 67–90
Wiklund C, Karlsson B (1984) Egg size variation in satyrid butterflies: adaptive vs. historical “Bauplan”, and mechanistic explanations. Oikos 43:391–400
Wiklund C, Persson A (1983) Fecundity, egg weight variation and its relation to offspring fitness in the speckled wood butterfly, Pararge aegeria, or why don’t butterfly females lay more eggs? Oikos 40:53–63
Wiklund C, Persson A, Wickman PO (1983) Larval aestivation and direct development as alternative strategies in the speckled wood butterfly, Pararge aegeria, in Sweden. Ecol Entomol 8:233–238
Wiklund C, Kaitala A, Lindström V, Abenius J (1993) Polyandry and its effect on female reproduction in the green-veined white butterfly, Pieris napi. Behav Ecol Sociobiol 33:25–34
Wiklund C, Kaitala A, Wedell N (1998) Decoupling of reproductive rates and parental expenditure in a polyandrous butterfly. Behav Ecol 9:20–25
Wiklund C, Karlsson B, Leimar O (2001) Sexual conflict and cooperation in butterfly reproduction: a comparative study of polyandry and female fitness. Proc R Soc Lond B 268:1661–1667
Acknowledgements
Two anonymous referees provided valuable comments on the manuscript. This study was supported by a grant of the University of Antwerp (GOA 15R/3942).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Wedell
Rights and permissions
About this article
Cite this article
Lauwers, K., Van Dyck, H. The cost of mating with a non-virgin male in a monandrous butterfly: experimental evidence from the speckled wood, Pararge aegeria . Behav Ecol Sociobiol 60, 69–76 (2006). https://doi.org/10.1007/s00265-005-0142-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0142-4