Abstract
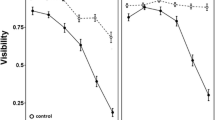
Tadpoles can alter their behavior, morphology, and life history in response to habitat change. Although chemical signals from conspecifics or predators play an important role in tadpole habitat assessment, little is known about the role of visual cues and the extent to which tadpoles rely on their vision for intraspecific social assessment. The aim of our experiments was to determine whether larval anurans use visual images of other tadpoles as indicators of density and to analyze how, and to what extent, images of conspecifics alone affect tadpole development, growth, and behavior. To assess this, we raised both Rana sylvatica and Bufo americanus tadpoles in aquaria with either quarter- or half-mirrored walls. Both physically increased density and increased density simulated with mirrors decreased tadpole growth and developmental rates, and increased activity in Rana tadpoles. Bufo tadpoles did not significantly alter their growth and development in response to visually increased density. Only true, i.e., physically, increased density had an effect on growth and activity in Bufo tadpoles. Our data show that images of conspecifics are used as visual cues by Rana tadpoles and can induce phenotypically plastic changes in several traits. This response to visual cues is taxon-specific.



Similar content being viewed by others
References
Arendt JD (2003) Reduced burst speed is a cost of rapid growth in anuran tadpoles: problems of autocorrelation and interferences about growth rates. Funct Ecol 7:328–334
Blaustein AR, O’Hara RK (1982) Kin recognition in Rana cascadae tadpoles: maternal and paternal effects. Anim Behav 30:1151–1157
Blaustein AR, Waldman B (1992) Kin recognition in anuran amphibians. Anim Behav 44:207–221
Blaustein AR, Walls SC (1995) Aggregation and kin recognition. In: Heatwole H, Sullivan BK (eds) Amphibian biology 2, social behaviour. Surrey Beatty, pp 568–602
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes in identification. Herpetologica 16:183–190
Hamilton WD (1971) Geometry for the selfish herd. J Theor Biol 31:295–311
Hobson ES (1979) Aggregation as a defense against predators in aquatic and terrestrial environments. In: Reese RS, Lighter FJ (eds) Contrasts in behavior. Wiley, New York, pp 219–234
Kats LB, Petranka JW, Sih A (1988) Antipredator defenses and the persistence of amphibian larvae with fishes. Ecology 69:1865–1870
Lannoo MJ (1999) Integration: nervous and sensory systems. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, pp 149–169
Licht LE (1967) Growth inhibition in crowded tadpoles: intraspecific and interspecific effects. Ecology 48:736–745
Petranka JW, Kats LB, Sih A (1987) Predator–prey interaction among fish and larval amphibians: use of chemical cues to detect predatory fish. Anim Behav 35:420–425
Relyea RA (2001) The lasting effects of adaptive plasticity: predator-induced tadpoles become long-legged frogs. Ecology 82:1947–1955
Relyea RA (2002) Competitor-induced plasticity in tadpoles: consequences, cues, and connections to predator-induced plasticity. Ecol Monogr 72:523–540
Relyea RA, Hoverman JT (2003) The impact of larval predators and competitors on the morphology and fitness of juvenile tree frogs. Oecologia 134:596–604
Relyea RA, Werner EE (2005) Morphological plasticity of four larval anurans distributed along an environmental gradient. Copeia 178–190
Rennie MD, Collins NC, Shuter BJ, Rajotte JW, Couture P (2005) A comparison of methods for estimating activity costs of wild fish populations: more active fish observed to grow slower. Can J Fish Aquat Sci 62:767–780
Rot-Nikcevic I, Denver RJ, Wassersug RJ (2005) The influence of visual and tactile stimulation on growth and metamorphosis in anuran larvae. Funct Ecol (in press)
SAS Institute (2001) SAS/STAT version 8.2. SAS Institute, Cary
Semlitsch RD, Caldwell JP (1982) Effects of density on growth, metamorphosis, and survivorship in tadpoles of Scaphiopus holbrooki. Ecology 63:905–911
Semlitsch RD, Reyer HU (1992) Modification of anti-predator behaviour in tadpoles by environmental conditioning. J Anim Ecol 61:353–360
Smith DC, Van Buskirk J (1995) Phenotypic design, plasticity, and ecological performance in two tadpole species. Am Nat 145:211–233
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–585
StatSoft (1997) STATISTICA for Windows, version 5.0. StatSoft, Tulsa
vS. Hoff K (1988) Evasive maneuvers of tadpoles and frogs in complex environments. Am Zool 28:155A
vS. Hoff K, Blaustein AR, McDiarmid RW, Altig R (1999) Behavior: Interactions and their consequences. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, pp 215–239
Waldman B (1982) Sibling association among schooling toad tadpoles: field evidence and implications. Anim Behav 30:700–713
Waldman B (1986) Chemical ecology of kin recognition in anuran amphibians. In: Duvall D, Muller-Schwarze D, Silverstein RM (eds) Chemical signals in vertebrates 4. Plenum, New York, pp 225–242
Wassersug RJ (1973) Aspects of social behavior in anuran larvae. In: Vial JH (ed) Evolutionary biology of the anurans. University of Missouri Press, Columbia, pp 273–297
Wassersug RJ, Hessler CM (1971) Tadpole behaviour: aggregation in larval Xenopus laevis. Anim Behav 19:386–389
Wassersug RJ, Lum AM, Potel MJ (1981) An analysis of school structure for tadpoles (Anura: Amphibia). Behav Ecol Sociobiol 9:15–22
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis. Science 182:1305–1314
Acknowledgements
We thank Kerri Oseen for her help with collecting egg clutches and editorial suggestions. Rick Relyea, Bruce Waldman, David Skelly, and Marco Dadda provided critical comments on the manuscript. This work was supported by the Natural Sciences and Engineering Research Council of Canada (awarded to I.R.N. and R.J.W.) and by an I.W. Killam Memorial Scholarship (to I.R.N.). The experiments conducted herein comply with the current law of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Christensen-Dalsgaard
An erratum to this article can be found at http://dx.doi.org/10.1007/s00265-006-0186-0
Rights and permissions
About this article
Cite this article
Rot-Nikcevic, I., Taylor, C.N. & Wassersug, R.J. The role of images of conspecifics as visual cues in the development and behavior of larval anurans. Behav Ecol Sociobiol 60, 19–25 (2006). https://doi.org/10.1007/s00265-005-0133-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0133-5