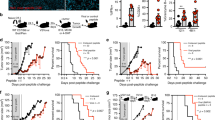
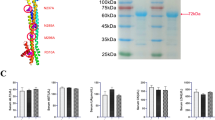
Abstract
Glioblastoma multiforme (GBM) is among the most aggressive, treatment-resistant cancers, and despite standard of care surgery, radiation and chemotherapy, is invariably fatal. GBM is marked by local and systemic immunosuppression, contributing to resistance to existing immunotherapies that have had success in other tumor types. Memory T cells specific for previous infections reside in tissues throughout the host and are capable of rapid and potent immune activation. Here, we show that virus-specific memory CD8 + T cells expressing tissue-resident markers populate the mouse and human glioblastoma microenvironment. Reactivating virus-specific memory T cells through intratumoral delivery of adjuvant-free virus-derived peptide triggered local immune activation. This delivery translated to antineoplastic effects, which improved survival in a murine glioblastoma model. Our results indicate that virus-specific memory T cells are a significant part of the glioblastoma immune microenvironment and may be leveraged to promote anti-tumoral immunity.






Similar content being viewed by others
Data Accessability
RNAseq data are deposited to the GEO database under the accession code GSE185029.
Change history
18 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00262-022-03168-7
References
Yu MW, Quail DF (2021) Immunotherapy for glioblastoma: current progress and challenge. Front Immunol 12:676301. https://doi.org/10.3389/fimmu.2021.676301
Lim M, Xia Y, Bettegowda C, Weller M (2018) Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15:422–442
Rosato PC, Wijeyesinghe S, Stolley JM et al (2019) Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat Commun 10:567. https://doi.org/10.1038/s41467-019-08534-1
Scheper W, Kelderman S, Fanchi LF et al (2018) Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med 359:1350–1394
Chiou S-H, Tseng D, Reuben A et al (2021) Global analysis of shared T cell specificities in human non-small cell lung cancer enables HLA inference and antigen discovery. Immunity 54:586-602.e8. https://doi.org/10.1016/j.immuni.2021.02.014
Duhen T, Duhen R, Montler R et al (2018) Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun 9:56
Andersen RS, Thrue CA, Junker N et al (2012) Dissection of T-cell antigen specificity in human melanoma. Cancer Res 72:1642–1650. https://doi.org/10.1158/0008-5472.CAN-11-2614
Simoni Y, Becht E, Fehlings M et al (2018) Bystander CD8 + T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557:575
Schenkel JM, Fraser KA, Vezys V, Masopust D (2013) Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol 14:509–513. https://doi.org/10.1038/ni.2568
Rosato PC, Beura LK, Masopust D (2017) Tissue resident memory T cells and viral immunity. Curr Opin Virol 22:44–50
Rosato PC, Wijeyesinghe S, Stolley JM, Masopust D (2020) Integrating resident memory into T cell differentiation models. Curr Opin Immunol 63:35–42. https://doi.org/10.1016/j.coi.2020.01.001
Schenkel JM, Fraser KA, Beura LK et al (2014) Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346:98–101. https://doi.org/10.1126/science.1254536
Ariotti S, Hogenbirk MA, Dijkgraaf FE et al (2014) T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346:101–105. https://doi.org/10.1126/science.1254803
Dhodapkar MV, Dhodapkar KM (2020) Tissue-resident memory-like T cells in tumor immunity: clinical implications. Semin Immunol 49:101415. https://doi.org/10.1016/j.smim.2020.101415
Vasquez JC, Huttner A, Zhang L et al (2017) SOX2 immunity and tissue resident memory in children and young adults with glioma. J Neurooncol 134:41–53. https://doi.org/10.1007/s11060-017-2515-8
Khan N, Shariff N, Cobbold M et al (2002) Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 169:1984–1992. https://doi.org/10.4049/jimmunol.169.4.1984
Szabo PA, Miron M, Farber DL (2019) Location, location, location: tissue resident memory T cells in mice and humans. Sci Immunol 4:eaas9673. https://doi.org/10.1126/sciimmunol.aas9673
Davies EJ, Dong M, Gutekunst M et al (2015) Capturing complex tumour biology in vitro: histological and molecular characterisation of precision cut slices. Sci Rep 5:17187
Beura LK, Hamilton SE, Bi K et al (2016) Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532:512–516
Wakim LM, Woodward-Davis A, Liu R et al (2012) The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 189:1201305–1203471
Wijeyesinghe S, Beura LK, Pierson MJ et al (2021) Expansible residence decentralizes immune homeostasis. Nature. https://doi.org/10.1038/s41586-021-03351-3
Hawke S, Stevenson PG, Freeman S, Bangham CR (1998) Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med 187:1575–1582
Urban SL, Jensen IJ, Shan Q et al (2020) Peripherally induced brain tissue–resident memory CD8 + T cells mediate protection against CNS infection. Nat Immunol 21:938–949. https://doi.org/10.1038/s41590-020-0711-8
Nelson CE, Thompson EA, Quarnstrom CF et al (2019) Robust iterative stimulation with self-antigens overcomes CD8+ T cell tolerance to self- and tumor antigens. Cell Rep 28:3092-3104.e5. https://doi.org/10.1016/j.celrep.2019.08.038
Szatmári T, Lumniczky K, Désaknai S et al (2006) Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci 97:546–553
Marumoto T, Tashiro A, Friedmann-Morvinski D et al (2009) Development of a novel mouse glioma model using lentiviral vectors. Nat Med 15:110–116. https://doi.org/10.1038/nm.1863
Khalsa JK, Cheng N, Keegan J et al (2020) Immune phenotyping of diverse syngeneic murine brain tumors identifies immunologically distinct types. Nat Commun 11:3912. https://doi.org/10.1038/s41467-020-17704-5
Casey KA, Fraser KA, Schenkel JM, et al (2012) Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol (Baltimore, Md: 1950) 188:4866–4875
Shiow LR, Rosen DB, Brdicková N et al (2006) CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540–544. https://doi.org/10.1038/nature04606
Millar DG, Ramjiawan RR, Kawaguchi K et al (2020) Antibody-mediated delivery of viral epitopes to tumors harnesses CMV-specific T cells for cancer therapy. Nat Biotechnol 38:420–425. https://doi.org/10.1038/s41587-019-0404-8
Sefrin JP, Hillringhaus L, Mundigl O et al (2019) Sensitization of tumors for attack by virus-specific CD8+ T-cells through antibody-mediated delivery of immunogenic T-cell epitopes. Front Immunol 10:1962. https://doi.org/10.3389/fimmu.2019.01962
Rahman M, Dastmalchi F, Karachi A, Mitchell D (2018) The role of CMV in glioblastoma and implications for immunotherapeutic strategies. Oncoimmunology 8:e1514921. https://doi.org/10.1080/2162402X.2018.1514921
Batich KA, Mitchell DA, Healy P et al (2020) Once, twice, three times a finding: reproducibility of dendritic cell vaccine trials targeting cytomegalovirus in glioblastoma. Clin Cancer Res 26:5297–5303. https://doi.org/10.1158/1078-0432.CCR-20-1082
Smith C, Lineburg KE, Martins JP et al (2020) Autologous CMV-specific T cells are a safe adjuvant immunotherapy for primary glioblastoma multiforme. J Clin Invest 130:6041–6053. https://doi.org/10.1172/JCI138649
Weathers S-P, Penas-Prado M, Pei B-L et al (2020) Glioblastoma-mediated immune dysfunction limits CMV-specific T cells and therapeutic responses: results from a phase I/II trial. Clin Cancer Res 26:3565–3577. https://doi.org/10.1158/1078-0432.CCR-20-0176
Beura LK, Mitchell JS, Thompson EA et al (2018) Intravital mucosal imaging of CD8+resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol 19:173–182
Cheng Y, Gunasegaran B, Singh HD et al (2021) Non-terminally exhausted tumor-resident memory HBV-specific T cell responses correlate with relapse-free survival in hepatocellular carcinoma. Immunity 54:1825-1840.e7. https://doi.org/10.1016/j.immuni.2021.06.013
Chongsathidkiet P, Jackson C, Koyama S et al (2018) Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med 24:1459–1468. https://doi.org/10.1038/s41591-018-0135-2
Ayasoufi K, Pfaller CK, Evgin L et al (2020) Brain cancer induces systemic immunosuppression through release of non-steroid soluble mediators. Brain 143:3629–3652. https://doi.org/10.1093/brain/awaa343
Akintola OO, Reardon DA (2021) The current landscape of immune checkpoint blockade in glioblastoma. Neurosurg Clin N Am 32:235–248. https://doi.org/10.1016/j.nec.2020.12.003
Liu P, Wang Y, Wang Y et al (2020) Effects of oncolytic viruses and viral vectors on immunity in glioblastoma. Gene Ther. https://doi.org/10.1038/s41434-020-00207-9
Anderson KG, Mayer-Barber K, Sung H et al (2014) Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9:209–222. https://doi.org/10.1038/nprot.2014.005
Steinert EM, Schenkel JM, Fraser KA et al (2015) Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161:737–749. https://doi.org/10.1016/j.cell.2015.03.031
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
Krämer A, Green J, Pollard J, Tugendreich S (2014) Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30:523–530. https://doi.org/10.1093/bioinformatics/btt703
Acknowledgements
The authors thank Dr. Ryan Langlois for PR8-gp33, the NIH Tetramer Facility for HLA-A*02:01 heavy chain plasmid, the Minnesota Supercomputing Institute for assistance with RNA sequencing, and University of Minnesota (UMN) BioNet for assistance with human samples.
Funding
This work was supported by a UMN SPORE Program Project Planning grant (DM, CCC), NCI 1R01CA238439 (DM), Humor to Fight the Tumor Foundation (JN), and support from the Norris Cotton Cancer Center, NCI 5P30CA023108-42 (PR). The authors have no relevant financial or non-financial interests to disclose. The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Mistake in figures 1 and 3.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ning, J., Gavil, N.V., Wu, S. et al. Functional virus-specific memory T cells survey glioblastoma. Cancer Immunol Immunother 71, 1863–1875 (2022). https://doi.org/10.1007/s00262-021-03125-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03125-w