Abstract
Background
Immune-related adverse events (irAEs) comprise a distinct spectrum of auto-inflammatory manifestations triggered due to immune checkpoint inhibitors (ICI). Current data on the association of irAEs with outcomes in NSCLC treated with nivolumab are limited.
Methods and objectives
We pooled data from 531 metastatic NSCLC patients from five centers treated with nivolumab after failing platinum-based chemotherapy. The primary objective was to investigate the relationship between irAEs with clinical benefit to nivolumab as well as to elucidate patterns of irAE-related ICI discontinuations and their impact on survival.
Results
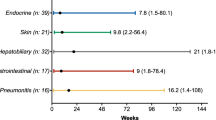
33.0% (173/531) of patients treated with nivolumab were noted to have an irAE. Patients with irAEs had a significantly longer median PFS [6.1 vs. 3.1 months, HR 0.68 95% CI (0.55–0.85); p = 0.001] and OS [14.9 vs. 7.4 months, HR 0.66 95% CI (0.52–0.82); p < 0.001)] compared to those without irAEs. In multivariate analysis, the presence of irAEs showed a significantly better PFS [HR 0.69, 95% CI (0.55–0.87); p = 0.002] and a trend for better OS [HR 0.62, 95% CI (0.55–1.03); p = 0.057]. Patients with permanent ICI discontinuation secondary to index irAE had a significantly shorter median PFS [2.3 vs. 6.6 months, HR 1.74 95% CI (1.06–2.80); p = 0.02] and median OS [3.6 vs. 17.6 months; HR 2.61 95% CI (1.61–4.21); p < 0.001] compared to those that did not have permanent ICI discontinuation.
Conclusions
Our pooled exploratory analysis demonstrates improved clinical benefit to nivolumab in NSCLC patients experiencing irAEs. We also observed negative impact of irAE-related treatment discontinuation on survival in this group of patients.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
28 April 2020
The original version of this article unfortunately contained a mistake. The second sentence of the section “irAEs and ICI efficacy” should read as.
Abbreviations
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein-4
- ECOG:
-
Eastern cooperate oncology group
- G:
-
Grade
- ICI:
-
Immune checkpoint inhibitors
- IrAE:
-
Immune-related adverse events
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death-1
- PFS:
-
Progression-free survival
- PS:
-
Performance status
References
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8(9):1069–1086. https://doi.org/10.1158/2159-8290.cd-18-0367
Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL et al (2019) Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res 25(15):4592–4602. https://doi.org/10.1158/1078-0432.CCR-18-1538
Vokes EE, Ready N, Felip E, Horn L, Burgio MA et al (2018) Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 29(4):959–965. https://doi.org/10.1093/annonc/mdy041
Young A, Quandt Z, Bluestone JA (2018) The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol Res 6(12):1445–1452. https://doi.org/10.1158/2326-6066.CIR-18-0487
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168. https://doi.org/10.1056/NEJMra1703481
Johnson DB, Chandra S, Sosman JA (2018) Immune checkpoint inhibitor toxicity in 2018 Immune checkpoint inhibitor toxicity immune checkpoint inhibitor toxicity. JAMA 320(16):1702–1703. https://doi.org/10.1001/jama.2018.13995
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol 36(17):1714–1768. https://doi.org/10.1200/jco.2017.77.6385
Reynolds KL, Cohen JV, Durbin S, Thomas M, Dougan M et al (2018) Inpatient admissions related to immune-related adverse effects (irAE) among patients treated with immune checkpoint inhibitors for advanced malignancy: a tsunami is coming, but are we ready? J Clin Oncol 36(5_suppl):127. https://doi.org/10.1200/jco.2018.36.5_suppl.127
Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R et al (2017) Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 35(34):3807–3814. https://doi.org/10.1200/jco.2017.73.2289
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J et al (2016) Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375(18):1749–1755. https://doi.org/10.1056/NEJMoa1609214
Berner F, Bomze D, Diem S, Ali OH, Fassler M et al (2019) Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol 5(7):1043–1047. https://doi.org/10.1001/jamaoncol.2019.0402
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4(3):374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F et al (2019) Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer 20(4):237–247. https://doi.org/10.1016/j.cllc.2019.02.006
Kothari S, Bagley S, Aggarwal C, Bauml J, Alley E et al (2017) P3.02c-029 immune-related adverse events and their effect on outcomes in patients (pts) with non-small cell lung cancer (NSCLC) treated with nivolumab: topic: IT. J Thorac Oncol 12(1):1290. https://doi.org/10.1016/j.jtho.2016.11.1824
Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA et al (2018) Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non–small-cell lung cancer. Clin Lung Cancer 19(6):e893–e900. https://doi.org/10.1016/j.cllc.2018.08.008
Zimmermann S, Peters S, Owinokoko T, Gadgeel SM (2018) Immune checkpoint inhibitors in the management of lung cancer. Am Soc Clin Oncol Educ Book 38:682–695. https://doi.org/10.1200/edbk_201319
Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG et al (2019) Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 145(2):479–485. https://doi.org/10.1007/s00432-018-2805-3
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Rapisuwon S, Izar B, Batenchuk C, Avila A, Mei S et al (2019) Exceptional response and multisystem autoimmune-like toxicities associated with the same T cell clone in a patient with uveal melanoma treated with immune checkpoint inhibitors. J Immuno Ther Cancer 7(1):61. https://doi.org/10.1186/s40425-019-0533-0
Das R, Bar N, Ferreira M, Newman AM, Zhang L et al (2018) Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 128(2):715–720. https://doi.org/10.1172/JCI96798
Naidoo J, Cappelli L, Lipson EJ, Forde PM, Sharfman WH et al (2018) A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J Clin Oncol 36(15_suppl):6538. https://doi.org/10.1200/jco.2018.36.15_suppl.6538
Nishino M, Hatabu H, Hodi FS, Ramaiya NH (2017) Drug-related pneumonitis in the era of precision cancer therapy. JCO Precis Oncol 1:1–12. https://doi.org/10.1200/po.17.00026
Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS et al (2018) Pneumonitis in non–small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol 13(12):1930–1939. https://doi.org/10.1016/j.jtho.2018.08.2035
Hofman P (2019) Is the onset of adverse effects of immunotherapy always bad news for the patients…?-certainly not. Ann Transl Med 7(1):5. https://doi.org/10.21037/atm.2019.01.14
Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G et al (2019) Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 145(2):511–521. https://doi.org/10.1007/s00432-018-2819-x
Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S et al (2019) Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol 37(30):2730–2737. https://doi.org/10.1200/JCO.19.00318
Spigel DR, McLeod M, Hussein MA, Waterhouse DM, Einhorn L et al (2017) 1297ORandomized results of fixed-duration (1-year) vs continuous nivolumab in patients (pts) with advanced non-small cell lung cancer (NSCLC). Ann Oncol. https://doi.org/10.1093/annonc/mdx380.002
Passaro A, Spitaleri G, Gyawali B, de Marinis F (2019) Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol 37(22):1863–1867. https://doi.org/10.1200/JCO.18.02118
Park W, Kwon D, Saravia D, Desai A, Vargas F et al (2018) Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin Lung Cancer 19(3):280–288. https://doi.org/10.1016/j.cllc.2017.12.007
Passaro A, Spitaleri G, Gyawali B, Marinis F (2018) Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. https://doi.org/10.1200/jco.18.02118
Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME et al (2018) Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 6(9):1093–1099. https://doi.org/10.1158/2326-6066.CIR-17-0755
Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M et al (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.1022
Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E et al (2019) Efficacy of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer needing treatment interruption because of adverse events: a retrospective multicenter analysis. Clinical Lung Cancer 20(1):e97–e106. https://doi.org/10.1016/j.cllc.2018.09.005
Acknowledgements
We thank the contributing centers as well as ECU IRB. We also thank the clinical research coordinators from ECU (Ms. Sue Ann Joyner and Ms. Susan Eubanks) for facilitating data sharing.
Funding
No relevant funding was required in conducting this study.
Author information
Authors and Affiliations
Contributions
ARN was involved in the acquisition, analysis, interpretation of data acquired from all institutions and drafting of the manuscript. ARN, CC, and MH were involved in the clinical annotation of data from East Carolina University. BR was involved in assisting in some aspects of the conceptual design of the study and data interpretation. BR, DHO, and VF were involved in critical revisions of the manuscript content. BR, AG, YT, JS, DHO, SKP, JB, VF, and WP were involved in collection of patient data from respective institutions. GAO, RC, SS, GDLL, CC, and PW were involved in the clinical care of patients from respective institutions. MM and GAO assisted in revising the manuscript drafts. ARN had full access to data from all participating institutions and assumes full responsibility for the integrity and accuracy of the analysis. All authors had access and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no potential conflicts of interest directly or indirectly relevant to the conduct or analysis of this study.
Ethical approval
The primary IRB approval was obtained from East Carolina Univ IRB (UMCIRB 15-001400). All participating centers had secured their respective IRB approval to collect their institutional data. To permit the sharing of de-identified data, a data use agreement was signed between ECU and the outside institution providing de-identified data. Patient consent was not required as part of the IRB approved studies since no identifiable information was shared or used for analysis or publication. All this was done in accordance with the regulations covered by the respective institutional IRBs.
Informed consent
No identifiable patient information has been published warranting individual consent from patients. The requirement for individual patient informed consent for the purpose of data collection and publication was waived as per the regulations covered under the respective institutional IRBs (East Carolina Univ, Ohio State Univ, Sendai Kousei Hospital, Univ of Perugia, University of Miami).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naqash, A.R., Ricciuti, B., Owen, D.H. et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother 69, 1177–1187 (2020). https://doi.org/10.1007/s00262-020-02536-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02536-5