Abstract
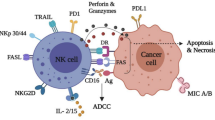
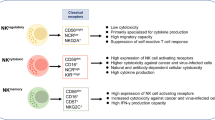
Natural killer (NK) cells are critically involved in anti-tumor immunity by targeting tumor cells. In this study, we show that intratumoral NK cells from NSCLC patients expressed elevated levels of the immune checkpoint receptor PD-1 on their cell surface. In contrast to the expression of activating receptors, PD-1+ NK cells co-expressed more inhibitory receptors compared to PD-1− NK cells. Intratumoral NK cells were less functional compared to peripheral NK cells, and this dysfunction correlated with PD-1 expression. Tumor cells expressing PD-L1 inhibited the functionality of PD-1+ NK cells in ex vivo models and induced PD-1 clustering at the immunological synapse between NK cells and tumor cells. Notably, treatment with PD-1 blockade was able to reverse PD-L1-mediated inhibition of PD-1+ NK cells. Our findings highlight the therapeutic potential of PD-1+ NK cells in immune checkpoint blockade and could guide the development of NK cell-stimulating agents in combination with PD-1 blockade.




Similar content being viewed by others
Abbreviations
- ATCC:
-
American type culture collection
- DBM:
-
Department of biomedicine
- HD:
-
Healthy donor
- ICB:
-
Immune checkpoint blockade
- KIR:
-
Killer immunoglobulin-like receptor
- LAG-3:
-
Lymphocyte activation gene-3
- NSCLC:
-
Non-small cell lung cancer
- NK:
-
Natural killer cell
- ORF:
-
Open reading frame
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death 1 ligand 1
- TIGIT:
-
T-cell immunoreceptor with Ig and ITIM domains
- TIL:
-
Tumor-infiltrating lymphocyte
- TIM-3:
-
T-cell immunoglobulin and mucin 3
References
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E et al (2015) Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med. 373(2):123–35. https://doi.org/10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csöszi T, Fülöp A et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C et al (2018) Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378(22):2093–2104. https://doi.org/10.1056/NEJMoa1801946
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. https://doi.org/10.1056/NEJMoa1801005
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301. https://doi.org/10.1056/NEJMoa1716948
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051. https://doi.org/10.1056/NEJMoa1810865
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R et al (2018) Overall survival with durvalumab after chemoradiotherapy in Stage III NSCLC. N Engl J Med 379(24):2342–2350. https://doi.org/10.1056/NEJMoa1809697
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn M-J et al (2019) Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the Phase I KEYNOTE-001 Study. J Clin Oncol. https://doi.org/10.1200/jco.19.00934
Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ (2015) Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8 + T cells. J Exp Med 212(7):1125–1137. https://doi.org/10.1084/jem.20142237
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A et al (2014) Genetic basis for clinical response to CTLA-4 blockade in Melanoma. N Engl J Med 371(23):2189–2199. https://doi.org/10.1056/NEJMoa1406498
Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF et al (2018) STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant Lung Adenocarcinoma. Cancer Discov 8(7):822–835. https://doi.org/10.1158/2159-8290.CD-18-0099
Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C et al (2018) MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med. 10(450):3342. https://doi.org/10.1126/scitranslmed.aar3342
López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L (2017) Control of metastasis by NK cells. Cancer Cell 32(2):135–154. https://doi.org/10.1016/j.ccell.2017.06.009
Morvan MG, Lanier LL (2016) NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16(1):7–19. https://doi.org/10.1038/nrc.2015.5
Voskoboinik I, Smyth MJ, Trapani JA (2006) Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol 6(12):940–952. https://doi.org/10.1038/nri1983
Screpanti V, Wallin RPA, Grandien A, Ljunggren H-G (2005) Impact of FASL-induced apoptosis in the elimination of tumor cells by NK cells. Mol Immunol 42(4):495–499. https://doi.org/10.1016/j.molimm.2004.07.033
Fauriat C, Long EO, Ljunggren H-G, Bryceson YT (2010) Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115(11):2167–2176. https://doi.org/10.1182/blood-2009-08-238469
Guillerey C, Smyth MJ (2016) NK cells and cancer immunoediting. Curr Top Microbiol Immunol 395:115–145. https://doi.org/10.1007/82_2015_446
Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean M-C, Riquet M et al (2013) Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 19(15):4079–4091. https://doi.org/10.1158/1078-0432.CCR-12-3847
Sconocchia G, Arriga R, Tornillo L, Terracciano L, Ferrone S, Spagnoli GC (2012) Melanoma cells inhibit NK cell functions-letter. Cancer Res 72(20):5428–5429. https://doi.org/10.1158/0008-5472.CAN-12-1181
Delahaye NF, Rusakiewicz S, Martins I, Ménard C, Roux S, Lyonnet L et al (2011) Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 17(6):700–707. https://doi.org/10.1038/nm.2366
Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P et al (2011) Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 71(16):5412–5422
Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G et al (2008) Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16 - cells and display an impaired capability to kill tumor cells. Cancer 112(4):863–75. https://doi.org/10.1002/cncr.23239
Hsu KC, Chida S, Geraghty DE, Dupont B (2002) The killer cell immunoglobulin-like receptor (KIR) genomic region: gene-order, haplotypes and allelic polymorphism. Immunol Rev 190(1):40–52. https://doi.org/10.1034/j.1600-065X.2002.19004.x
Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC et al (2001) Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 19:197–223
Trefny MP, Rothschild SI, Uhlenbrock F, Rieder D, Kasenda B, Stanczak MA et al (2019) A variant of a killer cell immunoglobulin-like receptor is associated with resistance to PD-1 blockade in lung cancer. Clin Cancer Res 25(10):3026–3034. https://doi.org/10.1158/1078-0432.CCR-18-3041
da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK et al (2014) Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res 2(5):410–422. https://doi.org/10.1158/2326-6066.CIR-13-0171
Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D et al (2017) Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol. 139(1):335–346. https://doi.org/10.1016/j.jaci.2016.04.025
Liu X, Hou M, Liu Y (2017) TIGIT, a novel therapeutic target for tumor immunotherapy. Immunol Invest 46(2):172–182
Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L (2014) Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol 44(6):1582–1592. https://doi.org/10.1002/eji.201344272
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R et al (2011) Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 121(9):3609–22. https://doi.org/10.1172/jci45816
Gill S, Vasey AE, De Souza A, Baker J, Smith AT, Kohrt HE et al (2012) Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood. 119(24):5758–68. https://doi.org/10.1182/blood-2012-03-415364
Choppa PC, Vojdani A, Tagle C, Andrin R, Magtoto L (1998) Multiplex PCR for the detection of Mycoplasma fermentans, M. hominis and M. penetrans in cell cultures and blood samples of patients with chronic fatigue syndrome. Mol Cell Probes. 12(5):301–8
Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E et al (2008) Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther 16(4):718–725. https://doi.org/10.1038/MT.2008.5
Thommen DS, Schreiner J, Muller P, Herzig P, Roller A, Belousov A et al (2015) Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 3(12):1344–1355. https://doi.org/10.1158/2326-6066.CIR-15-0097
Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C et al (2017) Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 36(44):6143–6153. https://doi.org/10.1038/onc.2017.209
Quatrini L, Wieduwild E, Escaliere B, Filtjens J, Chasson L, Laprie C et al (2018) Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat Immunol 19(9):954–962. https://doi.org/10.1038/s41590-018-0185-0
Guillerey C, Huntington ND, Smyth MJ (2016) Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17(9):1025–1036. https://doi.org/10.1038/ni.3518
Benson DM, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B et al (2010) The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116(13):2286–2294. https://doi.org/10.1182/blood-2010-02-271874
Wiesmayr S, Webber SA, Macedo C, Popescu I, Smith L, Luce J et al (2012) Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur J Immunol 42(2):541–550. https://doi.org/10.1002/eji.201141832
MacFarlane AW, Jillab M, Plimack ER, Hudes GR, Uzzo RG, Litwin S et al (2014) PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer Immunol Res 2(4):320–331. https://doi.org/10.3892/etm.2018.5788
Beldi-Ferchiou A, Lambert M, Dogniaux S, Vély F, Vivier E, Olive D et al (2016) PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget. https://doi.org/10.18632/oncotarget.12150
Chiossone L, Vienne M, Kerdiles YM, Vivier E (2017) Natural killer cell immunotherapies against cancer: checkpoint inhibitors and more. Semin Immunol 31:55–63. https://doi.org/10.1016/j.smim.2017.08.003
Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G et al (2019) Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 177(7):1701–1713.e16. https://doi.org/10.1016/J.CELL.2019.04.041
Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C et al (2018) Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 49(6):1148–1161.e7. https://doi.org/10.1016/j.immuni.2018.09.024
Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ et al (2018) A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat Med 24(8):1178–1191. https://doi.org/10.1038/s41591-018-0085-8
Zimmer J, Donato L, Hanau D, Cazenave J, Tongio M, Moretta A et al (1998) Activity and phenotype of natural killer cells in peptide transporter (TAP)-deficient patients (Type I bare lymphocyte syndrome). J Exp Med 187(1):117–122. https://doi.org/10.1084/JEM.187.1.117
Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA et al (2006) Human NK cell education by inhibitory receptors for MHC class I. Immunity 25(2):331–342. https://doi.org/10.1016/j.immuni.2006.06.013
Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault M-C, Trevino TN et al (2018) Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. https://doi.org/10.1172/JCI99317
Laughney AM, Hu J, Campbell NR, Bakhoum SF, Setty M, Lavallée V-P et al (2020) Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat Med 26(2):1–11. https://doi.org/10.1038/s41591-019-0750-6
Acknowledgement
We thank the FACS Core Facility of the DBM of the University of Basel for sorting cells used in this study. Moreover, we thank Prof. Dr. Baum and Prof. Dr. Axel Schambach (Medizinische Hochschule Hannover, Germany) for providing the pRRL.PPT.SFFV.EGFP.pre expression vector. We thank Dr. Ana Luisa Pinto Correia and Priska Auf der Maur for critical input on the manuscript. We also thank all the patients that allowed the use of their material and made this work possible.
Funding
This work was supported by grants from the Swiss National Science Foundation (320030_162575 to Alfred Zippelius) and a Research Fund of the University of Basel (to Franziska Uhlenbrock).
Author information
Authors and Affiliations
Contributions
FU, AZ and MPT conceived the idea for the study. FU, MPT and AZ interpreted the data, made the figures and wrote the manuscript. MPT, MK, MAS, FU and AZ planned the experiments. MPT, MK, MS and PH performed and analyzed the experiments. DL, MW and SS provided samples. HL and AZ collected the clinical data and ethical board approvals.
Corresponding authors
Ethics declarations
Conflict of interest
Heinz Läubli and Alfred Zippelius received research funding from Bristol-Myers Squibb. Alfred Zippelius received consulting/advisor fees from Bristol-Myers Squibb, Merck Sharp& Dohme, Hoffmann–La Roche, NBE Therapeutics, Secarna, ACM Pharma and Hookipa and maintains further non-commercial research agreements with Secarna, Hookipa, Roche and Beyondsprings. The authors declare that there are no other conflicts of interest.
Ethical approval and ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethikkommission Nordwestschweiz, Study Approval Number EK321/10) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study for the use of their specimens in research and publication. This article does not contain any studies with animals performed by any of the authors.
Cell line authentication
K562 cells (ATCC CCL-243) and HEK293T cells (ATCC CRL-3216) were purchased from ATCC, which provided detailed cell line authentication documentation. NA8-Mel was kindly provided by Dr. Romero (University of Lausanne) and their authenticity was confirmed by HLA-A2 positivity and cell morphology. NK92 cells (ATCC CRL-2407) were kindly provided by Dr. Bentires-Alj (University of Basel, Switzerland) and authenticity was certified by ATCC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trefny, M.P., Kaiser, M., Stanczak, M.A. et al. PD-1+ natural killer cells in human non-small cell lung cancer can be activated by PD-1/PD-L1 blockade. Cancer Immunol Immunother 69, 1505–1517 (2020). https://doi.org/10.1007/s00262-020-02558-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02558-z