Abstract
Background
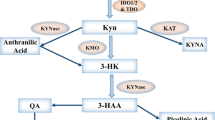
We previously found that PD-L1 expression is increased on tumor cells following vaccination treatments that lead to increased tumor-specific T cells that secrete IFNγ. Indoleamine 2,3-dioxygenase (IDO) is another IFNγ inducible gene that has potent immunosuppressive effects. There have been reports of IDO expression in prostate cancer; however, it is unknown whether IDO expression might similarly increase in prostate tumors following T-cell-based immunotherapy.
Methods
Blood samples from normal male blood donors (n = 12) and patients with different stages of prostate cancer (n = 89), including patients with metastatic, castration-resistant prostate cancer treated with a DNA vaccine and/or pembrolizumab, were evaluated for IDO activity by kynurenine and tryptophan levels. Metastatic tissue biopsies obtained pre- and post-treatments were evaluated for IDO expression. IDO suppression of vaccine-induced T-cell function was assessed by ELISPOT.
Results
Overall, IDO activity was increased in patients with more advanced prostate cancer. This activity, and IDO expression as detected immunohistochemically, increased following treatment with either a DNA vaccine encoding the prostatic acid phosphatase (PAP) tumor antigen or PD-1 blockade with pembrolizumab. Increased IDO activity after treatment was associated with the absence of clinical effect, as assessed by lack of PSA decline following treatment. Increased antigen-specific T-cell response, as measured by IFNγ release, to the vaccine target antigen was detected following in vitro stimulation of peripheral blood cells with 1-methyltryptophan.
Conclusions
These findings suggest that IDO expression is a mechanism of immune evasion used by prostate cancer and that future clinical trials using T-cell-based immune strategies might best include IDO inhibition.




Similar content being viewed by others
Abbreviations
- 1-MT:
-
1-methyltryptophan
- CTLA-4:
-
Cytolytic T-lymphocyte antigen 4
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DNA:
-
Deoxyribonucleic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- ELISPOT:
-
Enzyme-linked immunosorbent spot assay
- FFPE:
-
Formalin-fixed paraffin-embedded
- IDO:
-
Indoleamine 2,3-dioxygenase
- IF:
-
Immunofluorescent
- IFNγ:
-
Interferon-gamma
- IHC:
-
Immunohistochemistry
- IRB:
-
Institutional review board
- Kyn:
-
Kynurenine
- LC/MS:
-
Liquid chromatography/mass spectrometry
- mCRPC:
-
Metastatic, castration-resistant prostate cancer
- MDSC:
-
Myeloid-derived suppressor cell
- PAP:
-
Prostatic acid phosphatase
- PBMC:
-
Peripheral blood mononuclear cells
- PD-(L)1:
-
Programmed death-1 (ligand)
- PSA:
-
Prostate-specific antigen
- PSMA:
-
Prostate-specific membrane antigen
- pTVG-HP:
-
DNA vaccine-encoding human prostatic acid phosphatase
- TIL:
-
Tumor-infiltrating lymphocyte
- TRAMP:
-
Transgenic adenocarcinoma of mouse prostate
- trp:
-
Tryptophan
References
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274. https://doi.org/10.1038/nm934
Croitoru-Lamoury J, Lamoury FM, Caristo M, Suzuki K, Walker D, Takikawa O, Taylor R, Brew BJ (2011) Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS One 6:e14698. https://doi.org/10.1371/journal.pone.0014698
Mbongue JC, Nicholas DA, Torrez TW, Kim NS, Firek AF, Langridge WH (2015) The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines 3:703–729. https://doi.org/10.3390/vaccines3030703
Munn DH, Mellor AL (2016) IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol 37:193–207. https://doi.org/10.1016/j.it.2016.01.002
Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, Wolchok JD (2015) Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep 13:412–424. https://doi.org/10.1016/j.celrep.2015.08.077
Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP (2013) Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210:1389–1402. https://doi.org/10.1084/jem.20130066
Komiya T, Huang CH (2018) Updates in the clinical development of epacadostat and other indoleamine 2,3-dioxygenase 1 inhibitors (IDO1) for human cancers. Front Oncol 8:423. https://doi.org/10.3389/fonc.2018.00423
Kallberg E, Wikstrom P, Bergh A, Ivars F, Leanderson T (2010) Indoleamine 2,3-dioxygenase (IDO) activity influence tumor growth in the TRAMP prostate cancer model. Prostate 70:1461–1470. https://doi.org/10.1002/pros.21181
Feder-Mengus C, Wyler S, Hudolin T et al (2008) High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. Eur J Cancer 44:2266–2275. https://doi.org/10.1016/j.ejca.2008.05.023
Kolijn K, Verhoef EI, Smid M, Bottcher R, Jenster GW, Debets R, van Leenders G (2018) Epithelial-mesenchymal transition in human prostate cancer demonstrates enhanced immune evasion marked by IDO1 expression. Cancer Res 78:4671–4679. https://doi.org/10.1158/0008-5472.CAN-17-3752
Banzola I, Mengus C, Wyler S et al (2018) Expression of indoleamine 2,3-dioxygenase induced by IFN-gamma and TNF-alpha as potential biomarker of prostate cancer progression. Front Immunol 9:1051. https://doi.org/10.3389/fimmu.2018.01051
Rodriguez-Blanco G, Burgers PC, Dekker LJ, Vredenbregt-van den Berg MS, Ijzermans JN, Schenk-Braat EA, Jenster G, Luider TM (2014) Serum kynurenine/tryptophan ratio is not a potential marker for detecting prostate cancer. Clin Biochem 47:1347–1348. https://doi.org/10.1016/j.clinbiochem.2014.05.001
Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG (2015) PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol Res 3:946–955. https://doi.org/10.1158/2326-6066.CIR-14-0206
Rekoske BT, Olson BM, McNeel DG (2016) Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology 5:e1165377. https://doi.org/10.1080/2162402X.2016.1165377
Zahm CD, Colluru VT, McNeel DG (2017) Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8 + T cells. Cancer Immunol Res 5:630–641. https://doi.org/10.1158/2326-6066.CIR-16-0374
McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, Liu G (2018) Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 9:25586–25596. https://doi.org/10.18632/oncotarget.25387
Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H (1991) Induction of indoleamine 2,3-dioxygenase in human cells in vitro. Adv Exp Med Biol 294:505–509
Colluru VT, Zahm CD, McNeel DG (2016) Mini-intronic plasmid vaccination elicits tolerant LAG3 + CD8 + T cells and inferior antitumor responses. Oncoimmunology 5:e1223002. https://doi.org/10.1080/2162402x.2016.1223002
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
McNeel DG, Becker JT, Eickhoff JC et al (2014) Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin Cancer Res 20:3692–3704. https://doi.org/10.1158/1078-0432.ccr-14-0169
Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH (2007) Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res 67:792–801. https://doi.org/10.1158/0008-5472.CAN-06-2925
Brown ZJ, Yu SJ, Heinrich B et al (2018) Indoleamine 2,3-dioxygenase provides adaptive resistance to immune checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-018-2190-4
Long GV, Dummer R, Hamid O et al (2019) Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol 20:1083–1097. https://doi.org/10.1016/S1470-2045(19)30274-8
Jha GG, Gupta S, Tagawa ST, Koopmeiners JS, Vivek S, Dukdek AZ, Cooley SA, Blazar BR, Miller JS (2017) A phase II randomized, double-blind study of sipuleucel-T followed by IDO pathway inhibitor, indoximod, or placebo in the treatment of patients with metastatic castration resistant prostate cancer (mCRPC). In: 2017 ASCO Annual Meeting. pp. Abstract #3066. https://meetinglibrary.asco.org/record/145096/abstract
Acknowledgements
We are grateful to Dr. Robert Newton (Incyte) and Worldwide Clinical Trials for conducting analysis of tryptophan and kynurenine concentrations in blood samples, and to Dr. Glenn Liu for helpful comments on the manuscript.
Funding
This work was supported by the Prostate Cancer Foundation (2014 Movember-PCF Challenge Award) and by National Institutes of Health R01 CA219154 and P30 CA014520.
Author information
Authors and Affiliations
Contributions
CDZ conducted and analyzed laboratory studies described; DGM designed the study and oversaw analysis; all authors contributed to the writing and approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Douglas G. McNeel has ownership interest, has received research support, and serves as consultant to Madison Vaccines, Inc. which has licensed intellectual property related to this content. None of the other authors have relevant potential conflicts of interest.
Ethical approval
Long-term use of blood samples collected from research subjects who had previously consented for remaining samples to be used for immunology-related research was approved on 11/21/2016 under University of Wisconsin IRB protocol 2013-0126-CR004 as a minimal risk protocol, not requiring additional patient consent.
Informed consent
Samples were collected under University of Wisconsin IRB-approved protocols, and all patients gave written, informed consent for remaining samples to be used for research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zahm, C.D., Johnson, L.E. & McNeel, D.G. Increased indoleamine 2,3-dioxygenase activity and expression in prostate cancer following targeted immunotherapy. Cancer Immunol Immunother 68, 1661–1669 (2019). https://doi.org/10.1007/s00262-019-02394-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02394-w