Abstract
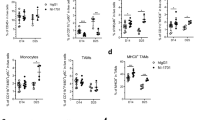
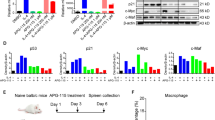
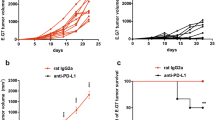
Although a role of PD-L1 in the suppression of anti-tumor immunity and its value as a predictive biomarker has been suggested by various preclinical and clinical studies, the precise mechanisms how PD-L1 and PD-L2, another ligand of PD-1, regulate anti-tumor immunity in the tumor microenvironment are yet to be fully explored. Here, we address this issue using PD-L1-deficient tumor cells, PD-L1-knockout (KO) mice, anti-PD-L1 monoclonal antibody (mAb), and anti-PD-L2 mAb. Firstly, PD-L1-deficient or competent tumor cells were inoculated into wild-type or PD-L1-KO mice. Results of tumor growth and mouse survival indicated that both tumor- and host-derived PD-L1 are functional to suppress anti-tumor immunity, while the former contributes predominantly than the latter. Experiments using bone marrow (BM) chimeric mice, generated by transferring PD-L1-KO BM cells into wild-type mice or vice versa, further suggested that PD-L1 expressed on BM-derived hematopoietic cells mediates the suppressive effects on anti-tumor immunity. Secondly, anti-PD-L2 mAb treatment demonstrated a profound synergy with anti-PD-L1 mAb therapy, whereas anti-PD-L2 mAb alone hardly induced any anti-tumor effects, suggesting that PD-L2’s function becomes evident when the effects of PD-L1 are abrogated by anti-PD-L1 mAb. Consistent with this notion, PD-L2 expression was upregulated on tumor-associated macrophages (TAM) when mice were treated with anti-PD-L1 mAb. Taken together, our study elucidated the importance of PD-L1 associated with tumor cells and non-tumor host cells, particularly BM-derived hematopoietic cells, as well as PD-L2 inducibly expressed on TAM in the suppression of anti-tumor immunity in the tumor microenvironment.






Similar content being viewed by others
Abbreviations
- ATCC:
-
American Type Culture Collection
- BM:
-
Bone marrow
- i.p.:
-
Intraperitoneally
- KO:
-
Knockout
- mAb:
-
Monoclonal antibody
- mAbs:
-
Monoclonal antibodies
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death-ligand 1
- PD-L2:
-
Programmed cell death-ligand 2
- s.c.:
-
Subcutaneously
- TAM:
-
Tumor-associated macrophages
References
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454. https://doi.org/10.1056/NEJMoa1200690
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366(26):2455–2465. https://doi.org/10.1056/NEJMoa1200694
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(6382):1350–1355. https://doi.org/10.1126/science.aar4060
Garber K (2015) Predictive biomarkers for checkpoints, first tests approved. Nat Biotechnol 33(12):1217–1218. https://doi.org/10.1038/nbt1215-1217
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813. https://doi.org/10.1056/NEJMoa1510665
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387(10031):1909–1920. https://doi.org/10.1016/S0140-6736(16)00561-4
Noguchi T, Ward JP, Gubin MM, Arthur CD, Lee SH, Hundal J, Selby MJ, Graziano RF, Mardis ER, Korman AJ, Schreiber RD (2017) Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res 5(2):106–117. https://doi.org/10.1158/2326-6066.CIR-16-0391
Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, Chiu H, Hong R, Grogan J, Javinal V, Yauch R, Irving B, Belvin M, Mellman I, Kim JM, Schmidt M (2017) Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 8:14572. https://doi.org/10.1038/ncomms14572
Kleinovink JW, Marijt KA, Schoonderwoerd MJA, van Hall T, Ossendorp F, Fransen MF (2017) PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology 6(4):e1294299. https://doi.org/10.1080/2162402X.2017.1294299
Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH (2017) PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 214(4):895–904. https://doi.org/10.1084/jem.20160801
Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, Liu X, Harrington SM, Guo J, Xin Y, Xiong Y, Nham K, Silvers W, Hao G, Sun X, Chen M, Hannan R, Qiao J, Dong H, Peng H, Fu YX (2018) PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Investig 128(2):580–588. https://doi.org/10.1172/JCI96061
Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, Szeliga W, Herbst R, Harms PW, Fecher LA, Vats P, Chinnaiyan AM, Lao CD, Lawrence TS, Wicha M, Hamanishi J, Mandai M, Kryczek I, Zou W (2018) Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Investig 128(2):805–815. https://doi.org/10.1172/JCI96113
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2(3):261–268. https://doi.org/10.1038/85330
Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H (2002) Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 169(10):5538–5545. https://doi.org/10.4049/jimmunol.169.10.5538
Messal N, Serriari NE, Pastor S, Nunes JA, Olive D (2011) PD-L2 is expressed on activated human T cells and regulates their function. Mol Immunol 48(15–16):2214–2219. https://doi.org/10.1016/j.molimm.2011.06.436
Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, Lunceford J, Cheng J, Chow LQM, Seiwert TY, Handa M, Tomassini JE, McClanahan T (2017) PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res 23(12):3158–3167. https://doi.org/10.1158/1078-0432.CCR-16-1761
Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Yokota SJ, Bankert RB (2007) Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol 178(9):5552–5562. https://doi.org/10.4049/jimmunol.178.9.5552
Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20(19):5064–5074. https://doi.org/10.1158/1078-0432.ccr-13-3271
Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L (2004) B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity 20(3):327–336
Clarke P, Mann J, Simpson JF, Rickard-Dickson K, Primus FJ (1998) Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res 58(7):1469–1477
Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65(3):1089–1096
Mazanet MM, Hughes CCW (2002) B7-H1 Is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol 169(7):3581–3588. https://doi.org/10.4049/jimmunol.169.7.3581
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4(1):71–78. https://doi.org/10.1038/nrc1256
Horlad H, Ma C, Yano H, Pan C, Ohnishi K, Fujiwara Y, Endo S, Kikukawa Y, Okuno Y, Matsuoka M, Takeya M, Komohara Y (2016) An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci 107(11):1696–1704. https://doi.org/10.1111/cas.13065
Liu J, Guan X, Ma X (2007) Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med 204(1):141–152. https://doi.org/10.1084/jem.20061440
Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS (2016) Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 7:10501. https://doi.org/10.1038/ncomms10501
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207(10):2187–2194. https://doi.org/10.1084/jem.20100643
Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA (2012) Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72(4):917–927. https://doi.org/10.1158/0008-5472.CAN-11-1620
Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL (2014) The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 26(6):923–937. https://doi.org/10.1016/j.ccell.2014.10.018
Acknowledgements
The authors thank Shunsuke Goto, Hiromi Kurosawa and Makiko Miyamoto for excellent technical assistance.
Funding
This study was supported by research funds from Grant-in-Aid for Scientific Research 16H02474 and Ono Pharmaceutical Inc.
Author information
Authors and Affiliations
Contributions
DU, NO, YS, and KA conducted experiments. TO, HY, ME, and KT guided the conduct of experiments. DU and KT wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Koji Tamada received research funds from Ono Pharmaceutical Inc. Other authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted (Yamaguchi University, Ube, Japan). Animal research was approved by the Institutional Animal Care and Use Committee of Yamaguchi University (animal research approval number: 14-001).
Animal source
Male or female 6 to 12-week-old wild-type C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). PD-L1-KO mice with a C57BL/6 background were kindly provided by Lieping Chen.
Cell line authentication
The MC38 mouse colon carcinoma cell line was kindly provided by F. James Primus. The 3LL mouse lung carcinoma cell line and the B16F10 mouse melanoma cell line were purchased from Japanese Collection of Research Bioresources Cell Bank and American Type Culture Collection (ATCC), respectively, who had authenticated them.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Umezu, D., Okada, N., Sakoda, Y. et al. Inhibitory functions of PD-L1 and PD-L2 in the regulation of anti-tumor immunity in murine tumor microenvironment. Cancer Immunol Immunother 68, 201–211 (2019). https://doi.org/10.1007/s00262-018-2263-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2263-4