Abstract
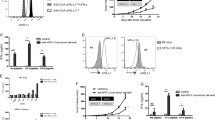
The expression of PD-L1 on tumor cells or within the tumor microenvironment has been associated with good prognosis and sustained clinical responses in immunotherapeutic regimens based on PD-L1/PD-1/CD80 immune checkpoint blockade. To look into the current controversy in cancer immunotherapy of the relative importance of PD-L1 expression on tumor cells versus non-tumor cells of the tumor microenvironment, a hematological mouse tumor model was chosen. By combining a genetic CRISPR/Cas9 and immunotherapeutic approach and using a syngeneic hematopoietic transplantable tumor model (E.G7-cOVA tumor cells), we demonstrated that dual blockade of PD-L1 interaction with PD-1 and CD80 enhanced anti-tumor immune responses that either delayed tumor growth or led to its complete eradication. PD-L1 expression on non-tumor cells of the tumor microenvironment was required for the promotion of tumor immune escape and its blockade elicited potent anti-tumor responses to PD-L1 WT and to PD-L1-deficient tumor cells. PD-L1+ tumors implanted in PD-L1-deficient mice exhibited delayed tumor growth independently of PD-L1 blockade. These findings emphasize that PD-L1 expression on non-tumor cells plays a major role in this tumor model. These observations should turn our attention to the tumor microenvironment in hematological malignancies because of its unappreciated contribution to create a conditioned niche for the tumor to grow and evade the anti-tumor immune response.






Similar content being viewed by others
Abbreviations
- APC:
-
Antigen-presenting cells
- ATCC:
-
American Type Culture Collection
- CD:
-
Cluster of differentiation
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen 4
- FCS:
-
Fetal calf serum
- ICB:
-
Immune checkpoint blockade
- mAb:
-
Monoclonal antibody
- MHC:
-
Major histocompatibility complex
- NK:
-
Natural killer
- PCR:
-
Polymerase chain reaction
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- PI:
-
Propidium iodide
- pLNs:
-
Peripheral lymph nodes
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- SFM:
-
Serum-free medium
- TCR:
-
T cell receptor
References
Ribatti D (2017) The concept of immune surveillance against tumors. The first theories. Oncotarget 8(4):7175–7180. https://doi.org/10.18632/oncotarget.12739
Dunn GP, Old LJ, Schreiber RD (2004) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21(2):137–148. https://doi.org/10.1016/j.immuni.2004.07.017
Finn OJ (2018) A believer’s overview of cancer immunosurveillance and immunotherapy. J Immunol (Baltimore, Md : 1950) 200(2):385–391. https://doi.org/10.4049/jimmunol.1701302
De Plaen E, Lurquin C, Van Pel A, Mariame B, Szikora JP, Wolfel T, Sibille C, Chomez P, Boon T (1988) Immunogenic (tum-) variants of mouse tumor P815: cloning of the gene of tum-antigen P91A and identification of the tum-mutation. Proc Natl Acad Sci USA 85(7):2274–2278
van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science (New York, NY) 254(5038):1643–1647
Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH (2016) Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol 34:539–573. https://doi.org/10.1146/annurev-immunol-032414-112049
Nicholas NS, Apollonio B (1863) Ramsay AG (2016) Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochem Biophys Acta 3:471–482. https://doi.org/10.1016/j.bbamcr.2015.11.003
Curran EK, Godfrey J, Kline J (2017) Mechanisms of immune tolerance in leukemia and lymphoma. Trends Immunol 38(7):513–525. https://doi.org/10.1016/j.it.2017.04.004
Upadhyay R, Hammerich L, Peng P, Brown B, Merad M, Brody JD (2015) Lymphoma: immune evasion strategies. Cancers 7(2):736–762. https://doi.org/10.3390/cancers7020736
Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P (1987) A new member of the immunoglobulin superfamily—CTLA-4. Nature 328(6127):267–270. https://doi.org/10.1038/328267a0
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science (New York, NY) 271(5256):1734–1736
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99(19):12293–12297
Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, Okazaki T, Honjo T (2009) PD-1-mediated suppression of IL-2 production induces CD8 + T cell anergy in vivo. J Immunol (Baltimore, Md : 1950) 182(11):6682–6689. https://doi.org/10.4049/jimmunol.0900080
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11(11):3887–3895
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science (New York, NY) 291(5502):319–322
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192(7):1027–1034
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2(3):261–268
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ (2007) Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27(1):111–122
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8(8):793–800
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571. https://doi.org/10.1038/nature13954
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. https://doi.org/10.1038/nrc3239
Gajewski TF, Louahed J, Brichard VG (2010) Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J (Sudbury, Mass) 16(4):399–403. https://doi.org/10.1097/PPO.0b013e3181eacbd8
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF (2013) Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 5(200):200ra116. https://doi.org/10.1126/scitranslmed.3006504
Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH (2017) PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 214(4):895–904. https://doi.org/10.1084/jem.20160801
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486–499. https://doi.org/10.1038/nri3862
De Sousa Linhares A, Leitner J, Grabmeier-Pfistershammer K, Steinberger P (2018) Not all immune checkpoints are created equal. Front Immunol 9:1909. https://doi.org/10.3389/fimmu.2018.01909
Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, Nyman J, Marjanovic ND, Kowalczyk MS, Wang C, Kurtulus S, Law T, Etminan Y, Nevin J, Buckley CD, Burkett PR, Buenrostro JD, Rozenblatt-Rosen O, Anderson AC, Regev A, Kuchroo VK (2018) Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 558(7710):454–459. https://doi.org/10.1038/s41586-018-0206-z
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44(5):989–1004. https://doi.org/10.1016/j.immuni.2016.05.001
Murray PJ, Smale ST (2012) Restraint of inflammatory signaling by interdependent strata of negative regulatory pathways. Nat Immunol 13(10):916–924. https://doi.org/10.1038/ni.2391
Annibali O, Crescenzi A, Tomarchio V, Pagano A, Bianchi A, Grifoni A, Avvisati G (2018) PD-1/PD-L1 checkpoint in hematological malignancies. Leuk Res 67:45–55. https://doi.org/10.1016/j.leukres.2018.01.014
Wilcox RA, Feldman AL, Wada DA, Yang ZZ, Comfere NI, Dong H, Kwon ED, Novak AJ, Markovic SN, Pittelkow MR, Witzig TE, Ansell SM (2009) B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood 114(10):2149–2158. https://doi.org/10.1182/blood-2009-04-216671
Carbone FR, Moore MW, Sheil JM, Bevan MJ (1988) Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J Exp Med 167(6):1767–1779
Young L, Sung J, Stacey G, Masters JR (2010) Detection of Mycoplasma in cell cultures. Nat Protoc 5(5):929–934. https://doi.org/10.1038/nprot.2010.43
Sanjana NE, Shalem O, Zhang F (2014) Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11(8):783–784. https://doi.org/10.1038/nmeth.3047
Tsushima F, Iwai H, Otsuki N, Abe M, Hirose S, Yamazaki T, Akiba H, Yagita H, Takahashi Y, Omura K, Okumura K, Azuma M (2003) Preferential contribution of B7-H1 to programmed death-1-mediated regulation of hapten-specific allergic inflammatory responses. Eur J Immunol 33(10):2773–2782
Unkeless JC (1979) Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med 150(3):580–596
Nelson DJ, Mukherjee S, Bundell C, Fisher S, van Hagen D, Robinson B (2001) Tumor progression despite efficient tumor antigen cross-presentation and effective “arming” of tumor antigen-specific CTL. J Immunol (Baltimore, Md : 1950) 166(9):5557–5566. https://doi.org/10.4049/jimmunol.166.9.5557
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8(11):2281–2308. https://doi.org/10.1038/nprot.2013.143
Tang F, Zheng P (2018) Tumor cells versus host immune cells: whose PD-L1 contributes to PD-1/PD-L1 blockade mediated cancer immunotherapy? Cell Biosci 8:34. https://doi.org/10.1186/s13578-018-0232-4
Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, Szeliga W, Herbst R, Harms PW, Fecher LA, Vats P, Chinnaiyan AM, Lao CD, Lawrence TS, Wicha M, Hamanishi J, Mandai M, Kryczek I, Zou W (2018) Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Investig 128(2):805–815. https://doi.org/10.1172/jci96113
Teng MW, Ngiow SF, Ribas A, Smyth MJ (2015) Classifying cancers based on T-cell Infiltration and PD-L1. Can Res 75(11):2139–2145. https://doi.org/10.1158/0008-5472.can-15-0255
Noguchi T, Ward JP, Gubin MM, Arthur CD, Lee SH, Hundal J, Selby MJ, Graziano RF, Mardis ER, Korman AJ, Schreiber RD (2017) Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res 5(2):106–117. https://doi.org/10.1158/2326-6066.cir-16-0391
Kleinovink JW, Marijt KA, Schoonderwoerd MJA, van Hall T, Ossendorp F, Fransen MF (2017) PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology 6(4):e1294299. https://doi.org/10.1080/2162402x.2017.1294299
Zhang X, Cheng C, Hou J, Qi X, Wang X, Han P, Yang X (2019) Distinct contribution of PD-L1 suppression by spatial expression of PD-L1 on tumor and non-tumor cells. Cell Mol Immunol 16(4):392–400. https://doi.org/10.1038/s41423-018-0021-3
Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D’Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ (2017) T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545(7652):60–65. https://doi.org/10.1038/nature22079
Umezu D, Okada N, Sakoda Y, Adachi K, Ojima T, Yamaue H, Eto M, Tamada K (2019) Inhibitory functions of PD-L1 and PD-L2 in the regulation of anti-tumor immunity in murine tumor microenvironment. Cancer Immunol Immunother 68(2):201–211. https://doi.org/10.1007/s00262-018-2263-4
Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, Liu X, Harrington SM, Guo J, Xin Y, Xiong Y, Nham K, Silvers W, Hao G, Sun X, Chen M, Hannan R, Qiao J, Dong H, Peng H, Fu YX (2018) PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Investig 128(2):580–588. https://doi.org/10.1172/jci96061
Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, Chiu H, Hong R, Grogan J, Javinal V, Yauch R, Irving B, Belvin M, Mellman I, Kim JM, Schmidt M (2017) Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 8:14572. https://doi.org/10.1038/ncomms14572
Shi L, Chen S, Yang L, Li Y (2013) The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol 6(1):74. https://doi.org/10.1186/1756-8722-6-74
Sugiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, Okazaki T (2019) Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science (New York, NY) 364(6440):558–566. https://doi.org/10.1126/science.aav7062
Held W, Mariuzza RA (2011) Cis-trans interactions of cell surface receptors: biological roles and structural basis. Cell Mol Life Sci 68(21):3469–3478. https://doi.org/10.1007/s00018-011-0798-z
Chaudhri A, Xiao Y, Klee AN, Wang X, Zhu B, Freeman GJ (2018) PD-L1 binds to B7-1 only in cis on the same cell surface. Cancer Immunol Res 6(8):921–929. https://doi.org/10.1158/2326-6066.cir-17-0316
Zhao Y, Lee CK, Lin CH, Gassen RB, Xu X, Huang Z, Xiao C, Bonorino C, Lu LF, Bui JD, Hui E (2019) PD-L1:CD80 cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity 51(6):1059e1059–1073e1059. https://doi.org/10.1016/j.immuni.2019.11.003
Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, Ahmed R (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537(7620):417–421. https://doi.org/10.1038/nature19330
Ikemizu S, Gilbert RJ, Fennelly JA, Collins AV, Harlos K, Jones EY, Stuart DI, Davis SJ (2000) Structure and dimerization of a soluble form of B7-1. Immunity 12(1):51–60. https://doi.org/10.1016/s1074-7613(00)80158-2
Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM (2011) Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science (New York, NY) 332(6029):600–603. https://doi.org/10.1126/science.1202947
Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E (2005) Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol 60(2):174–182. https://doi.org/10.1007/s00239-004-0046-3
Doudna JA, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science (New York, NY) 346(6213):1258096. https://doi.org/10.1126/science.1258096
Ajina R, Zamalin D, Zuo A, Moussa M, Catalfamo M, Jablonski SA, Weiner LM (2019) SpCas9-expression by tumor cells can cause T cell-dependent tumor rejection in immunocompetent mice. Oncoimmunology 8(5):e1577127. https://doi.org/10.1080/2162402x.2019.1577127
Burnet FM (1970) The concept of immunological surveillance. Prog Exp Tumor Res 13:1–27
Thomas L (1982) On immunosurveillance in human cancer. Yale J Biol Med 55(3–4):329–333
Xiong H, Mittman S, Rodriguez R, Pacheco-Sanchez P, Moskalenko M, Yang Y, Elstrott J, Ritter AT, Muller S, Nickles D, Arenzana TL, Capietto AH, Delamarre L, Modrusan Z, Rutz S, Mellman I, Cubas R (2019) Coexpression of inhibitory receptors enriches for activated and functional CD8(+) T cells in murine syngeneic tumor models. Cancer Immunol Res. https://doi.org/10.1158/2326-6066.cir-18-0750
Moore MW, Carbone FR, Bevan MJ (1988) Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54(6):777–785
Dranoff G (2012) Experimental mouse tumour models: what can be learnt about human cancer immunology? Nat Rev Immunol 12(1):61–66. https://doi.org/10.1038/nri3129
Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L (2004) B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity 20(3):327–336. https://doi.org/10.1016/s1074-7613(04)00050-0
Funding
This work has been supported by Grant FIS PI# 1300029 (Fondo de Investigaciones Sanitarias, Ministry of Health, Spanish Government, and co-funded by European Union ERDF/ESF, “Investing in your future”), LE093U13 and Unit of Excellence Research UIC #012 (Department of Education of the Regional Government, Junta de Castilla y Leon) and Gerencia Regional de Salud (BIO/01/15) to JIRB. It was also funded by Miguel Servet National Grant (Health National Organization Research) CP12/03063, CPII17/00002 and FIS PI16/00002 (Instituto de Salud Carlos III and co-funded by European Union ERDF/ESF, “Investing in your future”), and Gerencia Regional de Salud GRS963/A/2014, GRS1142/A/2015 and GRS 1505/A/2017 to M.L.R.G. This work has been partially funded by the National Network CIBER-ONC (oncology research) CB16/12/00480.
Author information
Authors and Affiliations
Contributions
Jose-Ignacio Rodriguez-Barbosa and Maria-Luisa del Rio conceived the working hypothesis, performed the experiments, analyzed data and wrote the manuscript. Miyuki Azuma and JA Perez-Simon contributed with reagents, comments and suggestions. Gennadiy Zelinskyy made the in vivo experiments using PD-L1-deficient mice. All authors discussed the results, provided critical input and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Animal Welfare Committee of the University of Alcala de Henares (Madrid) in accordance with the European Guidelines for Animal Care and Use of Laboratory Animals approved all experiments with rodents (authorization # OH-UAH-2016/015).
Animal source
Eight- to 12-week-old female C57BL-6 J (B6, from Janvier Labs) and C57BL/6-Tg (TcraTcrb)1100Mjb/J (also known as OT-I mice) were used in this work. OT-I transgenic mice exhibit a rearranged TCR that recognizes OVA residues 257–264, SIINFEKL peptide in the context of H-2b [63]. These mice were kindly provided by Dr. David Sancho (CNIC, National Center for Cardiovascular Disease, Madrid). B6-background PD-L1−/− (B7-H1-KO) mice were originally generated by Lieping Chen [65].
Cell line authentication
The EL-4 cell line is a chemically induced lymphoma cell line from C57BL/6 mice. E.G7 is a transplantable cell line derived from EL-4 thymoma cells that were transfected with a plasmid carrying a cytoplasmic version of chicken ovalbumin (OVA). Both cell lines were kindly provided by Prof. Dr. Ignacio Melero (CIMA, Navarra, Spain), who obtained them from ATCC. No cell line authentication was necessary.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodriguez-Barbosa, JI., Azuma, M., Zelinskyy, G. et al. Critical role of PD-L1 expression on non-tumor cells rather than on tumor cells for effective anti-PD-L1 immunotherapy in a transplantable mouse hematopoietic tumor model. Cancer Immunol Immunother 69, 1001–1014 (2020). https://doi.org/10.1007/s00262-020-02520-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02520-z