Abstract
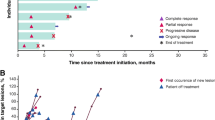
Treatment of metastatic melanoma remains challenging, despite a variety of new and promising immunotherapeutic and targeted approaches to therapy. New treatment options are still needed to improve long-term tumour control. We present a case series of seven patients with metastatic melanoma who were treated individually with the anti-CD20 antibody rituximab between July 2014 and July 2015. Two of the patients were treated in an adjuvant setting. All patients had already received a variety of treatments. During an induction phase, the administration of four cycles of weekly rituximab 375 mg/m2 body surface area was planned. After imaging, patients with stable disease continued therapy with rituximab 375 mg/m2 body surface area every 4 weeks up to a maximum of 24 weeks. Two patients experienced grade 2 infusion reactions during the first infusion. Otherwise, treatment was well tolerated and there were no grade 3 or 4 side effects. Staging after the induction phase showed stable disease in five patients, and two patients had progressive disease. Median progression-free survival was 6.3 months (95% CI 4.97–7.53), median overall survival was 14.7 months (95% CI 4.52–24.94), and one patient was still alive in December 2016. In conclusion, rituximab might be a therapeutic option for metastatic melanoma. However, further studies on rituximab among larger patient cohorts are warranted. Evaluation of therapy in an adjuvant setting or in combination with other systemic treatment might, therefore, be of particular interest.


Similar content being viewed by others
Abbreviations
- BRAF:
-
B-Raf proto-oncogene
- PFS:
-
Progression-free survival
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29. https://doi.org/10.3322/caac.21254
Grossmann KF, Margolin K (2015) Long-term survival as a treatment benchmark in melanoma: latest results and clinical implications. Ther Adv Med Oncol 7:181–191. https://doi.org/10.1177/1758834015572284
Maurer M, Somasundaram R, Herlyn M, Wagner SN (2012) Immunotargeting of tumor subpopulations in melanoma patients: A paradigm shift in therapy approaches. Oncoimmunology 1:1454–1456. https://doi.org/10.4161/onci.21357
Bittner M, Meltzer P, Chen Y et al (2000) Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406:536–540. https://doi.org/10.1038/35020115
Fang D, Nguyen TK, Leishear K et al (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65:9328–9337. https://doi.org/10.1158/0008-5472.CAN-05-1343
Chiaruttini G, Mele S, Opzoomer J, Crescioli S, Ilieva KM, Lacy KE, Karagiannis SN (2017) B cells and the humoral response in melanoma: The overlooked players of the tumor microenvironment. Oncoimmunology 6:e1294296. https://doi.org/10.1080/2162402X.2017.1294296
Ladanyi A, Kiss J, Mohos A et al (2011) Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother 60:1729–1738. https://doi.org/10.1007/s00262-011-1071-x
Garg K, Maurer M, Griss J, Bruggen MC, Wolf IH, Wagner C, Willi N, Mertz KD, Wagner SN (2016) Tumor-associated B cells in cutaneous primary melanoma and improved clinical outcome. Hum Pathol 54:157–164. https://doi.org/10.1016/j.humpath.2016.03.022
Saul L, Ilieva KM, Bax HJ et al (2016) IgG subclass switching and clonal expansion in cutaneous melanoma and normal skin. Sci Rep 6:29736. https://doi.org/10.1038/srep29736
Perricone MA, Smith KA, Claussen KA, Plog MS, Hempel DM, Roberts BL, St George JA, Kaplan JM (2004) Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother 27:273–281
Schwartz M, Zhang Y, Rosenblatt JD (2016) B cell regulation of the anti-tumor response and role in carcinogenesis. Immunother Cancer 4:40. https://doi.org/10.1186/s40425-016-0145-x
Egbuniwe IU, Mohamad MH, Karagiannis SN, Nestle FO, Lacy KE (2012) Interleukin-10-producing B-cell subpopulations in melanoma. British society for investigative dermatology meeting, Exeter. Br J Dermatol 166:e15–e40 (Poster P-21)
Somasundaram R, Zhang G, Fukunaga-Kalabis M et al (2017) Tumor-associated B-cells induce tumor heterogeneity and therapy resistance. Nat Commun 8:607. https://doi.org/10.1038/s41467-017-00452-4
Schmidt P, Abken H (2011) The beating heart of melanomas: a minor subset of cancer cells sustains tumor growth. Oncotarget 2:313–320. https://doi.org/10.18632/oncotarget.259
Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H (2011) Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci USA 108:2474–2479. https://doi.org/10.1073/pnas.1009069108
Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, Gajewski TF (2004) Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol 15:1109–1114. https://doi.org/10.1093/annonc/mdh280
Schlaak M, Schmidt P, Bangard C, Kurschat P, Mauch C, Abken H (2012) Regression of metastatic melanoma in a patient by antibody targeting of cancer stem cells. Oncotarget 3:22–30. https://doi.org/10.18632/oncotarget.437
Pinc A, Somasundaram R, Wagner C et al (2012) Targeting CD20 in melanoma patients at high risk of disease recurrence. Mol Ther 20:1056–1062. https://doi.org/10.1038/mt.2012.27
Song H, Su X, Yang K et al (2015) CD20 antibody-conjugated immunoliposomes for targeted chemotherapy of melanoma cancer initiating cells. J Biomed Nanotechnol 11:1927–1946
Westin JR, Chu F, Zhang M et al (2014) Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 15:69–77. https://doi.org/10.1016/S1470-2045(13)70551-5
Acknowledgements
The authors thank Hazel Davis for English revision.
Funding
No relevant funding.
Author information
Authors and Affiliations
Contributions
JKW analyzed clinical data and prepared the manuscript. MS and CB contributed to data acquisition and revised the manuscript. AHE was involved in manuscript proof-reading and finalization. JCH designed and coordinated the project, performed the immunohistochemical analyses and revised the manuscript. All authors had full access to all reported data and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors take responsibility for the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The institutional review board of the Medical Faculty of the University Hospital of Heidelberg (S-454/2015) approved the retrospective institutional patient data analysis. Guidelines from the Declaration of Helsinki were followed.
Informed consent
All patients gave written informed consent to individual treatment with rituximab. They were informed that therapy with rituximab is experimental and has not been approved for melanoma treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Winkler, J.K., Schiller, M., Bender, C. et al. Rituximab as a therapeutic option for patients with advanced melanoma. Cancer Immunol Immunother 67, 917–924 (2018). https://doi.org/10.1007/s00262-018-2145-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2145-9