Abstract
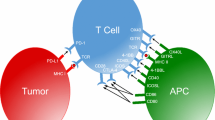
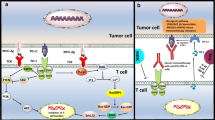
The discovery that antibody blockade of the T cell co-inhibitory receptor cytotoxic T lymphocyte-associated protein 4 (CTLA-4) can restore tumor immunity against many murine transplantable tumors leading to complete rejection of established cancer forever changed the field of immunotherapy. In more robust murine models as well as human cancer, however, CTLA-4 blockade alone can slow tumor growth and extend patient survival, but is rarely curative. Subsequent studies have revealed a large family of T cell immune checkpoint receptors which tumors engage to shield themselves from host immunity. As with CTLA-4, blockade of one of these additional inhibitory receptors, programmed death 1, has led to remarkable therapeutic responses against tumors of multiple lineages. Checkpoint monotherapy has demonstrated that durable, immune-mediated cures of established metastatic cancers are possible, yet the percentage of patients experiencing these outcomes remains low due to both redundant mechanisms of immune suppression in the tumor and limiting toxicity associated with some therapies. Thus, extending the curative potential of immunotherapy to a larger percentage of patients with a broader spectrum of malignancies will likely require combinations of co-inhibitory blockade and co-stimulatory activation designed to peel back multiple layers of tumor immune suppression while at the same time minimizing immune-mediated toxicity. As over a dozen T cell immune checkpoints and an additional dozen more co-stimulatory receptors have now been described, the challenge before us is to identify the most advantageous combinations of these agents based on the knowledge of their underlying biology and preclinical studies in murine tumor models.


Similar content being viewed by others
Abbreviations
- CAR:
-
Chimeric antigen receptor
- CSF1R:
-
Colony stimulating factor 1 receptor
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- EAE:
-
Experimental autoimmune encephalomyelitis
- FVAX:
-
B16-Flt3 ligand
- ICOS:
-
Inducible T cell co-stimulator
- IFN-γ:
-
Interferon gamma
- IRAE:
-
Immune-related adverse events
- MHC:
-
Major histocompatibility complex
- PBMC:
-
Peripheral blood mononuclear cells
- PD-1:
-
Programmed death 1
- RECIST:
-
Response Evaluation Criteria In Solid Tumors
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cells (CD4+FoxP3+)
References
Ehrlich P (1909) Über den jetzigen Stand der Chemotherapie. Ber Dtsch Chem Ges 42:17–47
Zinkernagel RM, Doherty PC (1974) Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature 251:547–548
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10:909–915
Wick M, Dubey P, Koeppen H, Siegel CT, Fields PE, Chen L, Bluestone JA, Schreiber H (1997) Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J Exp Med 186:229–238
Krummel MF, Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182:459–465
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734–1736
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895
Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L (1997) Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 3:682–685
Weinberg AD, Rivera MM, Prell R et al (2000) Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol 164:2160–2169
Moran AE, Kovacsovics-Bankowski M, Weinberg AD (2013) The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol 25:230–237
Croft M, Benedict CA, Ware CF (2013) Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 12:147–168
Podojil JR, Miller SD (2013) Targeting the B7 family of co-stimulatory molecules: successes and challenges. BioDrugs 27:1–13
Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA (1999) ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397:263–266
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW (1995) Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270:985–988
Nishimura H, Nose M, Hiai H, Minato N, Honjo T (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141–151
Nishimura H, Okazaki T, Tanaka Y et al (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319–322
Curran MA, Montalvo W, Yagita H, Allison JP (2010) PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 107:4275–4280
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ (2007) Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27:111–122
Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA (2013) Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res 19:997–1008
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. New Engl J Med 369:122–133
Sznol M, Kluger HM, Callahan MK et al (2014) Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). J Clin Oncol 32(5s Suppl):LBA9003
Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G (2013) Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 73:3591–3603
Callahan MK, Horak CE, Curran MA et al (2013) Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. J Clin Oncol 31(Suppl):3003
Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, Zhang Y, Zhao X, Weber JS (2012) Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J Transl Med 10:146
Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Allison JP, Sharma P (2013) Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res 1:229–234
Carthon BC, Wolchok JD, Yuan J et al (2010) Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 16:2861–2871
Fu T, He Q, Sharma P (2011) The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res 71:5445–5454
Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP (2014) Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med 211:715–725
Lee SW, Croft M (2009) 4-1BB as a therapeutic target for human disease. Adv Exp Med Biol 647:120–129
Wang C, Lin GH, McPherson AJ, Watts TH (2009) Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev 229:192–215
Li B, Lin J, Vanroey M, Jure-Kunkel M, Jooss K (2007) Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin Immunol 125:76–87
Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP (2013) Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med 210:743–755
Belcaid Z, Phallen JA, Zeng J et al (2014) Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One 9:e101764
Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP (2011) Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One 6:e19499
Kocak E, Lute K, Chang X et al (2006) Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res 66:7276–7284
Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu YX (2002) Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol 168:1457–1465
Kim YH, Choi BK, Shin SM et al (2011) 4-1BB triggering ameliorates experimental autoimmune encephalomyelitis by modulating the balance between Th17 and regulatory T cells. J Immunol 187:1120–1128
Callahan MK, Yang A, Tandon S et al (2011) Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J Clin Oncol 29(Suppl):2505
Vudattu NK, Waldron-Lynch F, Truman LA, Deng S, Preston-Hurlburt P, Torres R, Raycroft MT, Mamula MJ, Herold KC (2014) Humanized mice as a model for aberrant responses in human T cell immunotherapy. J Immunol 193:587–596
Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I (2010) Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol 37:508–516
Kvistborg P, Philips D, Kelderman S et al (2014) Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med 6:254ra128
Spranger S, Bao R, Gajewski TF (2014) Melanoma-intrinsic β-catenin signaling prevents T cell infiltration and anti-tumor immunity. J ImmunoTherapy Cancer 2(Suppl 3):O15
Gubin MM, Zhang X, Schuster H et al (2014) Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515:577–581
van Rooij N, van Buuren MM, Philips D et al (2013) Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 31:e439–e442
Acknowledgments
The work from our laboratory cited in this review was partially funded by a U.T. MD Anderson Moonshot Knowledge Gap award.
Conflict of interest
Dr. Curran has a research collaboration with Threshold Pharmaceuticals for which his laboratory has received funding, and an active collaboration with Astrazeneca in which compounds are provided free of charge. Dr. Curran also receives royalties from the patent “Methods and Compositions for Localized Secretion of anti-CTLA-4 Antibodies.” Dr. Ai has no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is a Focussed Research Review based on a presentation given at the Twelfth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 6th-8th May, 2014. It is part of a CII series of Focussed Research Reviews and meeting report.
Rights and permissions
About this article
Cite this article
Ai, M., Curran, M.A. Immune checkpoint combinations from mouse to man. Cancer Immunol Immunother 64, 885–892 (2015). https://doi.org/10.1007/s00262-014-1650-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1650-8