Abstract
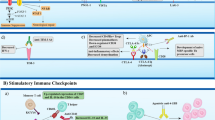
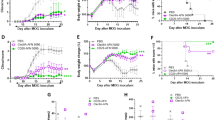
As more patient data is cross-referenced with animal models of disease, the primary focus on Th1 autoreactive effector cell function in autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis, has shifted towards the role of Th17 autoreactive effector cells and the ability of regulatory T cells (Treg) to modulate the pro-inflammatory autoimmune response. Therefore, the currently favored hypothesis is that a delicate balance between Th1/17 effector cells and Treg cell function is critical in the regulation of inflammatory autoimmune disease. An intensive area of research with regard to the Th1/17:Treg cell balance is the utilization of blockade and/or ligation of various co-stimulatory or co-inhibitory molecules, respectively, during ongoing disease to skew the immune response toward a more tolerogenic/regulatory state. Currently, FDA-approved therapies for multiple sclerosis patients are all aimed at the suppression of immune cell function. The other favored method of treatment is a modulation or deletion of autoreactive immune cells via short-term blockade of activating co-stimulatory receptors via treatment with fusion proteins such as CTLA4-Ig and CTLA4-FasL. Based on the initial success of CTLA4-Ig, there are additional fusion proteins that are currently under development. Examples of the more recently identified B7/CD28 family members are PD-L1, PD-L2, inducible co-stimulatory molecule-ligand (ICOS-L), B7-H3, and B7-H4, all of which may emerge as potential fusion protein therapeutics, each with unique, yet often overlapping functions. The expression of both stimulatory and inhibitory B7 molecules seems to play an essential role in modulating immune cell function through a variety of mechanisms, which is supported by findings that suggest each B7 molecule has developed its own indispensable niche in the immune system. As more data are generated, the diagnostic and therapeutic potential of the above B7 family-member-derived fusion proteins becomes ever more apparent. Besides defining the biology of these B7/CD28 family members in vivo, additional difficulty in the development of these therapies lies in maintaining the normal immune functions of recognition and reaction to non-self-antigens following viral or bacterial infection in the patient. Further complicating the clinical translation of these therapies, the mechanism of action identified for a particular reagent may depend upon the method of immune-cell activation and the subset of immune cells targeted in the study.


Similar content being viewed by others
References
Vidard L, Rock KL, Benacerraf B. Heterogeneity in antigen processing by different types of antigen-presenting cells. Effect of cell culture on antigen processing ability. J Immunol. 1992;149:1905–11.
Miller SD, McMahon EJ, Schreiner B, Bailey SL. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann NY Acad Sci. 2007;1103:179–91.
Robbie-Ryan M, Brown MA. The role of mast cells in allergy and autoimmunity. Curr Opin Immunol. 2002;14(6):728–33.
Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. High levels of a major histocompatibility complex II-self peptide complex on dendritic cells from the T cell areas of lymph nodes. J Exp Med. 1997;186(5):665–72.
Spack EG. Treatment of autoimmune diseases through manipulation of antigen presentation. Crit Rev Immunol. 1997;17(5–6):529–36.
Benacerraf B, Germain RN. The immune response genes of the major histocompatibility complex. Immunol Rev. 1978;38:70–119.
June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–6.
Aiello FB, Longo DL, Overton R, Takacs L, Durum SK. A role for cytokines in antigen presentation: IL-1 and IL-4 induce accessory functions of antigen-presenting cells. J Immunol. 1990;144(7):2572–81.
Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96.
Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, et al. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180(1):223–31.
Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203(4):1045–54.
Celada F, Klein G. Autonomy of H-2 genes in individual immunocytes. Nature. 1967;215(5106):1136–9.
Uhr JW, Finkelstein MS. Antibody formation. IV. Formation of rapidly and slowly sedimenting antibodies and immunological memory to bacteriophage phi-X 174. J Exp Med. 1963;117:457–77.
Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–6.
Adelstein S, Pritchard-Briscoe H, Anderson TA, Crosbie J, Gammon G, Loblay RH, et al. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991;251:1223–5.
Boitard C, Bendelac A, Richard MF, Carnaud C, Bach JF. Prevention of diabetes in nonobese diabetic mice by anti-I-A monoclonal antibodies: transfer of protection by splenic T cells. Proc Natl Acad Sci USA. 1988;85(24):9719–23.
Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435(7039):224–8.
Janeway CA Jr. The priming of helper T cells. Semin Immunol. 1989;1(1):13–20.
Zamvil SS, Mitchell DJ, Moore AC, Kitamura K, Steinman L, Rothbard JB. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–60.
Zinkernagel RM, Doherty PC. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177.
Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu Rev Neurosci. 2002;25:491–505.
Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53(1):27–42.
Damle NK, Klussman K, Linsley PS, Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992;148:1985–92.
Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–8.
Harding FA, McArthur J, Gross JA, Raulet D, Allison JP. CD28 mediated signalling costimulates murine T cells and prevents induction of anergy in T cell clones. Nature. 1992;356:607–9.
Norton SD, Zuckerman L, Urdahl KB, Shefner R, Miller J, Jenkins MK. The CD28 ligand, B7, enhances IL-2 production by providing a costimulatory signal to T cells. J Immunol. 1992;149:1556–61.
Seder RA, Germain RN, Linsley PS, Paul WE. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon gamma production. J Exp Med. 1994;179:299–304.
Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20(1):4–12.
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–4.
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–89.
Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116(5):1317–26.
Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156(1):5–7.
Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192(1):123–8.
Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157(8):3223–7.
Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995;181(1):381–6.
Bright JJ, Du C, Coon M, Sriram S, Klaus SJ. Prevention of experimental allergic encephalomyelitis via inhibition of IL-12 signaling and IL-12-mediated Th1 differentiation: an effect of the novel anti-inflammatory drug lisofylline. J Immunol. 1998;161(12):7015–22.
Ichikawa M, Koh CS, Inoue A, Tsuyusaki J, Yamazaki M, Inaba Y, et al. Anti-IL-12 antibody prevents the development and progression of multiple sclerosis-like relapsing-remitting demyelinating disease in NOD mice induced with myelin oligodendrocyte glycoprotein peptide. J Neuroimmunol. 2000;102(1):56–66.
Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169(12):7104–10.
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–8.
Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178(3):1341–8.
Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–63.
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–73.
Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–7.
Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199(11):1467–77.
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8.
Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161(1):72–87.
Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160(3):1212–8.
Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184(2):387–96.
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64.
Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–40.
Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166(6):3789–96.
Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201(8):1333–46.
Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–7.
Hall GL, Compston A, Scolding NJ. Beta-interferon and multiple sclerosis. Trends Neurosci. 1997;20(2):63–7.
Martin-Saavedra FM, Flores N, Dorado B, Eguiluz C, Bravo B, Garcia-Merino A, et al. Beta-interferon unbalances the peripheral T cell proinflammatory response in experimental autoimmune encephalomyelitis. Mol Immunol. 2007;44(14):3597–607.
Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, The Copolymer 1 Multiple Sclerosis Study Group, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology. 1995;45(7):1268–76.
Johnson KP. A review of the clinical efficacy profile of copolymer 1: new U.S. phase III trial data. J Neurol. 1996;243((4 Suppl. 1)):S3–7.
Dhib-Jalbut S. Glatiramer acetate (Copaxone) therapy for multiple sclerosis. Pharmacol Ther. 2003;98(2):245–55.
Ridge SC, Sloboda AE, McReynolds RA, Levine S, Oronsky AL, Kerwar SS. Suppression of experimental allergic encephalomyelitis by mitoxantrone. Clin Immunol Immnopathol. 1985;35:35–42.
Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64(8):1336–42.
O’Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, et al. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology. 2009;72(1):73–9.
Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A, et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol immunol. 2005;2(6):439–48.
Khalili K, White MK, Lublin F, Ferrante P, Berger JR. Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology. 2007;68(13):985–90.
Alegre ML, Tso JY, Sattar HA, Smith J, Desalle F, Cole M, et al. An anti-murine CD3 monoclonal antibody with a low affinity for Fc gamma receptors suppresses transplantation responses while minimizing acute toxicity and immunogenicity. J Immunol. 1995;155(3):1544–55.
Ben Amor A, Leite-De-Moraes MC, Lepault F, Schneider E, Machavoine F, Arnould A, et al. In vitro T cell unresponsiveness following low-dose injection of anti-CD3 MoAb. Clin Exp Immunol. 1996;103(3):491–8.
Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, et al. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174(8):4525–34.
Summers KL, O’Donnell JL, Williams LA, Hart DN. Expression and function of CD80 and CD86 costimulator molecules on synovial dendritic cells in chronic arthritis. Arthritis Rheum. 1996;39(8):1287–91.
Webb LM, Walmsley MJ, Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7–1 and B7–2. Eur J Immunol. 1996;26(10):2320–8.
Perrin PJ, Scott D, Quigley L, Albert PS, Feder O, Gray GS, et al. Role of B7:CD28/CTLA-4 in the induction of chronic relapsing experimental allergic encephalomyelitis. J Immunol. 1995;154(3):1481–90.
Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7–1 (CD80) and B7–2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998;161(6):2762–71.
Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, et al. B7–1 and B7–2 costimulatory molecules differentially activate the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18.
Perrin PJ, Scott D, Davis TA, Gray GS, Doggett MJ, Abe R, et al. Opposing effects of CTLA4-Ig and anti-CD80 (B7–1) plus anti-CD86 (B7–2) on experimental allergic encephalomyelitis. J Neuroimmunol. 1996;65(1):31–9.
Miller SD, Vanderlugt CL, Lenschow DJ, Pope JG, Karandikar NJ, Dal Canto MC, et al. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–45.
Vanderlugt CL, Karandikar NJ, Lenschow DJ, Dal Canto MC, Bluestone JA, Miller SD. Treatment with intact anti-B7-1 mAb during disease remission enhances epitope spreading and exacerbates relapses in R-EAE. J Neuroimmunol. 1997;79:113–8.
Kohm AP, Podojil JR, Williams JS, McMahon JS, Miller SD. CD28 regulates glucocorticoid-induced TNF receptor family-related gene (GITR) expression on CD4+ T cells via IL-2 dependent mechanisms. Cell Immunol. 2005;235:56–64.
Srinivasan M, Gienapp IE, Stuckman SS, Rogers CJ, Jewell SD, Kaumaya PT, et al. Suppression of experimental autoimmune encephalomyelitis using peptide mimics of CD28. J Immunol. 2002;169(4):2180–8.
Kantarci OH, Hebrink DD, Achenbach SJ, Atkinson EJ, Waliszewska A, Buckle G, et al. CTLA4 is associated with susceptibility to multiple sclerosis. J Neuroimmunol. 2003;134(1–2):133–41.
Linsley PS, Green JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7–1 (CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801.
Racke MK, Scott DE, Quigley L, Gray GS, Abe R, June CH, et al. Distinct roles for B7–1 (CD80) and B7–2 (CD86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:195–203.
Knoerzer DB, Karr RW, Schwartz BD, Mengle-Gaw LJ. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Invest. 1995;96(2):987–93.
Quattrocchi E, Walmsley M, Browne K, Williams RO, Marinova-Mutafchieva L, Buurman W, et al. Paradoxical effects of adenovirus-mediated blockade of TNF activity in murine collagen-induced arthritis. J Immunol. 1999;163(2):1000–9.
Podojil JR, Kohm AP, Miller SD. CD4+ T cell expressed CD80 regulates central nervous system effector function and survival during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:2948–58.
McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11(3):335–9.
Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42.
Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002;277(10):7766–75.
Gribben JG, Freeman GJ, Boussiotis VA, Rennert P, Jellis CL, Greenfield E, et al. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc Nat Acad Sci USA. 1995;92:811–5.
Boise LH, Noel PJ, Thompson CB. CD28 and apoptosis. Curr Opin Immunol. 1995;7(5):620–5.
Fallarino F, Fields PE, Gajewski TF. B7–1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med. 1998;188(1):205–10.
Plumas J, Chaperot L, Jacob MC, Molens JP, Giroux C, Sotto JJ, et al. Malignant B lymphocytes from non-Hodgkin’s lymphoma induce allogeneic proliferative and cytotoxic T cell responses in primary mixed lymphocyte cultures: an important role of co- stimulatory molecules CD80 (B7–1) and CD86 (B7–2) in stimulation by tumor cells. Eur J Immunol. 1995;25(12):3332–41.
Vyth-Dreese FA, Dellemijn TA, van Oostveen JW, Feltkamp CA, Hekman A. Functional expression of adhesion receptors and costimulatory molecules by fresh and immortalized B-cell non-Hodgkin’s lymphoma cells. Blood. 1995;85(10):2802–12.
Bhat S, Czuczman MS. Galiximab: a review. Exp Opin Biol Ther. 2010;10(3):451–8.
Fanale MA, Younes A. Monoclonal antibodies in the treatment of non-Hodgkin’s lymphoma. Drugs. 2007;67(3):333–50.
Vinjamaram S, Czuczman MS, Hernandez-Ilizaliturri FJ. The use of galiximab in non-Hodgkin lymphoma. Clin Lymphoma Myeloma. 2008;8(5):277–82.
Dakappagari N, Ho SN, Gascoyne RD, Ranuio J, Weng AP, Tangri S. CD80 (B7.1) is expressed on both malignant B cells and nonmalignant stromal cells in non-Hodgkin lymphoma. Cytom B Clin Cytom. 2012;82(2):112–9.
Martinez-Paniagua MA, Vega MI, Huerta-Yepez S, Baritaki S, Vega GG, Hariharan K, et al. Galiximab signals B-NHL cells and inhibits the activities of NF-kappaB-induced YY1- and snail-resistant factors: mechanism of sensitization to apoptosis by chemoimmunotherapeutic drugs. Mol Cancer Ther. 2012;11(3):572–81.
Podojil JR, Miller SD. Cross-linking of CD80 on CD4+ T cells activates a calcium-dependent signaling pathway. J Immunol. 2009;182(2):766–73.
Mauri D, Pichler WJ. Involvement of CD80 in the generation of CD4+ cytotoxic T cells. Immunol Res. 1996;15(2):126–40.
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5.
Schmidt EM, Wang CJ, Ryan GA, Clough LE, Qureshi OS, Goodall M, et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182(1):274–82.
Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–3.
Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4(+)CD25(+) regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169(9):4712–6.
McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168(12):5979–83.
Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199(11):1479–89.
Piccirillo CA, Tritt M, Sgouroudis E, Albanese A, Pyzik M, Hay V. Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice. Ann NY Acad Sci. 2005;1051:72–87.
Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–65.
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6.
O’Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193(1–2):1–11.
Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174(7):4407–14.
Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79(12):7860–7.
Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J Virol. 2008;82(14):6838–51.
Zelinskyy G, Kraft AR, Schimmer S, Arndt T, Dittmer U. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur J Immunol. 2006;36(10):2658–70.
Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, et al. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20(3):293–303.
He H, Messer RJ, Sakaguchi S, Yang G, Robertson SJ, Hasenkrug KJ. Reduction of retrovirus-induced immunosuppression by in vivo modulation of T cells during acute infection. J Virol. 2004;78(21):11641–7.
Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173(8):5008–20.
Richards MH, Getts MT, Podojil JR, Jin YH, Kim BS, Miller SD. Virus expanded regulatory T cells control disease severity in the Theiler’s virus mouse model of MS. J Autoimmun. 2011;36(2):142–54.
Olson JK, Croxford JL, Miller SD. Virus-induced autoimmunity: potential role of viruses in initiation, perpetuation, and progression of T cell-mediated autoimmune diseases. Viral Immunol. 2001;14(3):227–50.
Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9(4):246–58.
Lipton HL, Melvold R. Genetic analysis of susceptibility to Theiler’s virus-induced demyelinating disease in mice. J Immunol. 1984;132:1821–5.
Lyman MA, Myoung J, Mohindru M, Kim BS. Quantitative, not qualitative, differences in CD8(+) T cell responses to Theiler’s murine encephalomyelitis virus between resistant C57BL/6 and susceptible SJL/J mice. Eur J Immunol. 2004;34(10):2730–9.
Azoulay A, Brahic M, Bureau JF. FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler’s virus. J Virol. 1994;68(6):4049–52.
Clatch RJ, Melvold RW, Miller SD, Lipton HL. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TMEV-specific delayed-type hypersensitivity. J Immunol. 1985;135:1408–14.
Brahic M, Bureau JF. Genetics of susceptibility to Theiler’s virus infection. Bioessays. 1998;20(8):627–33.
Azoulay-Cayla A, Syan S, Brahic M, Bureau JF. Roles of the H-2D(b) and H-K(b) genes in resistance to persistent Theiler’s murine encephalomyelitis virus infection of the central nervous system. J Gen Virol. 2001;82(5):1043–7.
Azoulay-Cayla A, Dethlefs S, Perarnau B, Larsson-Sciard EL, Lemonnier FA, Brahic M, et al. H-2D(b-/-) mice are susceptible to persistent infection by Theiler’s virus. J Virol. 2000;74(12):5470–6.
Lipton HL, Melvold R, Miller SD, Dal Canto MC, Jensen K. Mutation of a major histocompatibility class I locus, H-2D, leads to an increased virus burden and disease susceptibility in Theiler’s virus-induced demyelinating disease. J Neurovirol. 1995;1:138–44.
Getts MT, Kim BS, Miller SD. Differential outcome of tolerance induction in naive versus activated Theiler’s virus epitope-specific CD8+ cytotoxic T cells. J Virol. 2007;81(12):6584–93.
Getts MT, Richards MH, Miller SD. A critical role for virus-specific CD8(+) CTLs in protection from Theiler’s virus-induced demyelination in disease-susceptible SJL mice. Virology. 2010;402(1):102–11.
Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, et al. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–6.
Katz-Levy Y, Neville KL, Girvin AM, Vanderlugt CL, Pope JG, Tan LJ, et al. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler’s virus-infected mice. J Clin Invest. 1999;104(5):599–610.
Kim PS, Ho GY, Prete PE, Furst DE. Safety and efficacy of abatacept in eight rheumatoid arthritis patients with chronic hepatitis B. Arthritis Care Res. 2012;64(8):1265–8.
Freeman GJ, Borriello F, Hodes RJ, Reiser H, Gribben JG, Ng JW, et al. Murine B7–2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993;178:2185–92.
Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257(5071):789–92.
Lenschow DJ, Su GH, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, et al. Expression and functional significance of an additional ligand for CTLA-4. Proc Nat Acad Sci USA. 1993;90:11054–8.
Montel AH, Bochan MR, Hobbs JA, Lynch DH, Brahmi Z. Fas involvement in cytotoxicity mediated by human NK cells. Cell Immunol. 1995;166(2):236–46.
Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85(12):3378–404.
Watanabe D, Suda T, Nagata S. Expression of Fas in B cells of the mouse germinal center and Fas-dependent killing of activated B cells. Int Immunol. 1995;7(12):1949–56.
Tanaka M, Suda T, Yatomi T, Nakamura N, Nagata S. Lethal effect of recombinant human Fas ligand in mice pretreated with Propionibacterium acnes. J Immunol. 1997;158(5):2303–9.
Tykocinski ML, Kaplan DR, Medof ME. Antigen-presenting cell engineering. The molecular toolbox. Am J Pathol. 1996;148(1):1–16.
Huang JH, Tykocinski ML. CTLA-4-Fas ligand functions as a trans signal converter protein in bridging antigen-presenting cells and T cells. Int Immunol. 2001;13(4):529–39.
Jin Y, Qu A, Wang GM, Hao J, Gao X, Xie S. Simultaneous stimulation of Fas-mediated apoptosis and blockade of costimulation prevent autoimmune diabetes in mice induced by multiple low-dose streptozotocin. Gene Ther. 2004;11(12):982–91.
Feng YG, Jin YZ, Zhang QY, Hao J, Wang GM, Xie SS. CTLA4-Fas ligand gene transfer mediated by adenovirus induce long-time survival of murine cardiac allografts. Transpl Proc. 2005;37(5):2379–81.
Paust S, Lu LR, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Nat Acad Sci USA. 2004;101(28):10398–403.
Weiss EM, Schmidt A, Vobis D, Garbi N, Lahl K, Mayer CT, et al. Foxp3-mediated suppression of CD95L expression confers resistance to activation-induced cell death in regulatory T cells. J Immunol. 2011;187(4):1684–91.
Johnson JG, Jenkins MK. Accessory cell-derived signals required for T cell activation. Immunol Rev. 1993;12:48–64.
Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48.
Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–47.
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22.
Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19(3):309–14.
Yasunami R, Bach JF. Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur J Immunol. 1988;18(3):481–4.
Kaifu T, Escaliere B, Gastinel LN, Vivier E, Baratin M. B7–H6/NKp30 interaction: a mechanism of alerting NK cells against tumors. CMLS. 2011;68(21):3531–9.
Joyce MG, Tran P, Zhuravleva MA, Jaw J, Colonna M, Sun PD. Crystal structure of human natural cytotoxicity receptor NKp30 and identification of its ligand binding site. Proc Natl Acad Sci USA. 2011;108(15):6223–8.
Li Y, Wang Q, Mariuzza RA. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7–H6. J Exp Med. 2011;208(4):703–14.
Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–503.
Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur J Immunol. 2008;38(10):2706–17.
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–29.
McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165(9):5035–40.
Hodgson R, Christiansen D, Ziolkowski A, Mouhtouris E, Simeonovic CJ, Ierino FL, et al. Prolonged xenograft survival induced by inducible costimulator-Ig is associated with increased forkhead box P3(+) cells. Transplantation. 2011;91(10):1090–7.
Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48.
Youngnak P, Kozono Y, Kozono H, Iwai H, Otsuki N, Jin H, et al. Differential binding properties of B7–H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307(3):672–7.
Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201(10):1531–41.
Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis: amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17(1):2–15.
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–44.
Cao Q, Wang Y, Zheng D, Sun Y, Lee VW, Zheng G, et al. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21(6):933–42.
Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, et al. Genomic organization and expression analysis of B7–H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171(9):4650–4.
Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7–H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–61.
Ichikawa M, Chen L. Role of B7–H1 and B7–H4 molecules in down-regulating effector phase of T-cell immunity: novel cancer escaping mechanisms. Front Biosci. 2005;10:2856–60.
Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, et al. B7–h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66(3):1570–5.
Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, et al. B7–h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11(5):1842–8.
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7–H3 and B7–H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–51.
Wang X, Hao J, Metzger DL, Mui A, Ao Z, Akhoundsadegh N, et al. Early treatment of NOD mice with B7–H4 reduces the incidence of autoimmune diabetes. Diabetes. 2011;60(12):3246–55.
Wang X, Hao J, Metzger DL, Ao Z, Meloche M, Verchere CB, et al. B7-H4 pathway in islet transplantation and beta-cell replacement therapies. J Transplant. 2011;2011:418902. (Epub 2011 Oct 13).
Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–92.
Acknowledgements
The authors are supported in part by US Public Health Service, National Institute of Health Grants, National Multiple Sclerosis Society, Amplimmune, Inc. and Compugen, Ltd. JRP and SDM are paid consultants for, recipients of a research grant from, and co-inventors on a patent application from Amplimmune, Inc., whose product AMP-110 (B7-H4-Ig) is discussed in this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Podojil, J.R., Miller, S.D. Targeting the B7 Family of Co-Stimulatory Molecules. BioDrugs 27, 1–13 (2013). https://doi.org/10.1007/s40259-012-0001-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-012-0001-6