Abstract
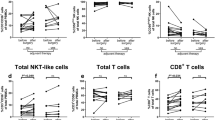
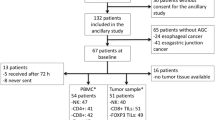
Immunotherapeutic approaches are emerging as promising new treatment options for patients with solid cancers. The host immune system in cancer patients is dysfunctional due to a number of reasons. The level of immunosuppression is variable at the time of diagnosis and depends on the particular cancer entity, stage, and prior anti-cancer therapies. For many cancer entities, the immune alterations of the respective patient population have not been further characterized even though a patient’s immunophenotype may be prognostic for the course of the disease or predictive for clinical/biological response to immunotherapy. In this study, we used flow cytometry to determine the phenotype of peripheral blood mononuclear cells (PBMCs) from 30 patients with heavily pre-treated, advanced adenocarcinoma of the stomach and gastro-esophageal junction. The frequencies and activation status of relevant immune effector populations were determined in PBMCs and compared to those of healthy individuals. This report provides comprehensive immune phenotyping data of a patient population with a high medical need.





Similar content being viewed by others
Abbreviations
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- CDC:
-
Complement-dependent cytotoxicity
- CIC:
-
Cancer Immunotherapy Consortium
- CIMT:
-
Association for Cancer Immunotherapy
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen-4
- CV:
-
Coefficients of variance
- DC:
-
Dendritic cell
- DMSO:
-
Dimethyl sulfoxide
- FDA:
-
Food and Drug Administration
- FoxP3:
-
Forkhead box protein 3
- HD:
-
Healthy donors
- IgG1:
-
Immunoglobulin G subclass I
- M:
-
Metastasis
- mAb:
-
Monoclonal antibody
- MDSCs:
-
Myeloid-derived suppressor cells
- N:
-
Node
- NK cells:
-
Natural killer cells
- n.s.:
-
Not significant
- P:
-
Patient
- PBMCs:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate-buffered saline
- PD-1:
-
Programmed death-1
- PDL-1:
-
Programmed death ligand-1
- RCC:
-
Renal cell carcinoma
- T:
-
Tumor
- Tregs:
-
Regulatory T cells
References
Kantoff PW, Higano CS, Shore ND et al (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422. doi:10.1056/NEJMoa1001294
Fong L, Small EJ (2008) Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol 26:5275–5283. doi:10.1200/JCO.2008.17.8954
Weber JS, Hamid O, Chasalow SD et al (2012) Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother 35:89–97. doi:10.1097/CJI.0b013e31823aa41c
Brahmer JR, Tykodi SS, Chow LQ et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465. doi:10.1056/NEJMoa1200694
Arnould L, Gelly M, Penault-Llorca F et al (2006) Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 94:259–267. doi:10.1038/sj.bjc.6602930
Kurai J, Chikumi H, Hashimoto K et al (2007) Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res 13:1552–1561. doi:10.1158/1078-0432.CCR-06-1726
Bibeau F, Lopez-Crapez E, Di Fiore F et al (2009) Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol 27:1122–1129. doi:10.1200/JCO.2008.18.0463
Cartron G, Dacheux L, Salles G et al (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99:754–758
Musolino A, Naldi N, Bortesi B et al (2008) Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 26:1789–1796. doi:10.1200/JCO.2007.14.8957
Racila E, Link BK, Weng WK et al (2008) A polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphoma. Clin Cancer Res 14:6697–6703. doi:10.1158/1078-0432.CCR-08-0745
Braun DP, Harris JE (1981) Relationship of leukocyte numbers, immunoregulatory cell function, and phytohemagglutinin responsiveness in cancer patients. J Natl Cancer Inst 67:809–814
Hong WS, Kim CM, Lee JO, Kang TW, Yun TK, Kim CY (1990) Natural killer and lymphokine-activated killer activities in stomach cancer patients with special emphasis on the effect of 5-fluorouracil, adriamycin and mitomycin-C chemotherapy. Jpn J Clin Oncol 20:87–93
Hong WS, Min YI, Son YS, Hong SI (1995) Peripheral blood lymphocyte subsets in patients with stomach cancer. J Korean Med Sci 10:164–168
Dillman RO, Koziol JA, Zavanelli MI et al (1984) Immunoincompetence in cancer patients. Assessment by in vitro stimulation tests and quantification of lymphocyte subpopulations. Cancer 53:1484–1491
Kaszubowski PA, Husby G, Tung KS, Williams RC Jr (1980) T-lymphocyte subpopulations in peripheral blood and tissues of cancer patients. Cancer Res 40:4648–4657
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. doi:10.3322/caac.20107
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150. doi:10.1200/JCO.2005.05.2308
Parkin DM (2004) International variation. Oncogene 23:6329–6340. doi:10.1038/sj.onc.1207726
Schuler MH, Zvirbule Z, Lordick F et al (2013) Safety, tolerability and efficacy of the first-in-class antibody IMAB362 as evaluated in a phase I single dose and phase II multiple dose study in patients with advanced gastroesophageal adenocarcinomas. J Clin Oncol 31:Suppl; abstr 4080
Miyara M, Yoshioka Y, Kitoh A et al (2009) Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899–911. doi:10.1016/j.immuni.2009.03.019
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9:606–612
Li L, Wu CY (2008) CD4+ CD25+ Treg cells inhibit human memory gammadelta T cells to produce IFN-gamma in response to M tuberculosis antigen ESAT-6. Blood 111:5629–5636. doi:10.1182/blood-2008-02-139899
Wing JB, Sakaguchi S (2012) Multiple treg suppressive modules and their adaptability. Front Immunol 3:178. doi:10.3389/fimmu.2012.00178
Curiel TJ, Coukos G, Zou L et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949. doi:10.1038/nm1093
Liotta F, Gacci M, Frosali F et al (2011) Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int 107:1500–1506. doi:10.1111/j.1464-410X.2010.09555.x
Anz D, Mueller W, Golic M et al (2011) CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J Cancer 129:2417–2426. doi:10.1002/ijc.25902
Carreras J, Lopez-Guillermo A, Fox BC et al (2006) High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 108:2957–2964. doi:10.1182/blood-2006-04-018218
Curiel TJ (2007) Tregs and rethinking cancer immunotherapy. J Clin Invest 117:1167–1174. doi:10.1172/JCI31202
Walter S, Weinschenk T, Stenzl A et al (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 18:1254–1261. doi:10.1038/nm.2883
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181:5791–5802
Schmielau J, Finn OJ (2001) Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 61:4756–4760
Almand B, Clark JI, Nikitina E et al (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 166:678–689
Gallina G, Dolcetti L, Serafini P et al (2006) Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 116:2777–2790. doi:10.1172/JCI28828
Pohla H, Buchner A, Stadlbauer B et al (2013) High immune response rates and decreased frequencies of regulatory T cells in metastatic renal cell carcinoma patients after tumor cell vaccination. Mol Med 18:1499–1508. doi:10.2119/molmed.2012.00221
Solito S, Falisi E, Diaz-Montero CM et al (2011) A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 118:2254–2265. doi:10.1182/blood-2010-12-325753
Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW (2011) Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 60:1419–1430. doi:10.1007/s00262-011-1028-0
Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM (2000) In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA 97:2731–2736. doi:10.1073/pnas.050588297
Coca S, Perez-Piqueras J, Martinez D et al (1997) The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 79:2320–2328
Levy EM, Roberti MP, Mordoh J (2011) Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol 2011:676198. doi:10.1155/2011/676198
Lanier LL, Ruitenberg J, Bolhuis RL, Borst J, Phillips JH, Testi R (1988) Structural and serological heterogeneity of gamma/delta T cell antigen receptor expression in thymus and peripheral blood. Eur J Immunol 18:1985–1992. doi:10.1002/eji.1830181218
Lee AJ, Kim SG, Chae HD, Lee GH, Shin IH (2012) gammadelta T cells are increased in the peripheral blood of patients with gastric cancer. Clin Chim Acta 413:1495–1499. doi:10.1016/j.cca.2012.06.016
Meraviglia S, Eberl M, Vermijlen D et al (2010) In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol 161:290–297. doi:10.1111/j.1365-2249.2010.04167.x
Tokuyama H, Hagi T, Mattarollo SR et al (2008) V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs—rituximab and trastuzumab. Int J Cancer 122:2526–2534. doi:10.1002/ijc.23365
Dieli F, Vermijlen D, Fulfaro F et al (2007) Targeting human gamma}delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 67:7450–7457. doi:10.1158/0008-5472.CAN-07-0199
Roux A, Mourin G, Larsen M et al (2013) Differential impact of age and cytomegalovirus infection on the gammadelta T cell compartment. J Immunol 191:1300–1306. doi:10.4049/jimmunol.1202940
Acknowledgments
We wish to thank Helene Schroeder and Nicole Bidmon for sample processing and Daniela Kirsch and Richard Rae for their technical assistance with performing flow cytometry. We thank Marc Roller, Marlene Knippenberg, and Vilmos Posevitz for excellent scientific writing support. The majority of samples were provided by clinical trial centers represented by authors of this manuscript as part of their participation in the phase II clinical trial testing the safety and single-agent activity of the therapeutic antibody IMAB362. We wish to acknowledge also all investigators and clinical trial centers, who have provided single patient samples: Ulrike Helbig, Hospital Braunschweig, Wolff Schmiegel, University Hospital Bochum, Salah-Eddin Al-Batran, Hospital Nordwest Frankfurt, Joern Ruessel, University Hospital Halle a. d. Saale, Susanna Hegewisch-Becker, Hematologisch-Onkologische Praxis Eppendorf, Hamburg and Albrecht Hoffmeister, University Hospital Leipzig (all Germany).
Conflict of interest
M.-C. Kuehnle and Ö. Türeci are employees of Ganymed Pharmaceuticals AG; U. Sahin and Ö. Türeci are inventors of patents on claudin18.2 and hold stock of Ganymed Pharmaceuticals AG. U. Sahin is consultant of Ganymed Pharmaceuticals AG. S. Attig, C. M. Britten, H. Schulze-Bergkamen, F. Lordick, G. von Wichert, P. Thuss-Patience, A. Stein, M. Schuler, and F. Bassermann declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuehnle, MC., Attig, S., Britten, C.M. et al. Phenotyping of peripheral blood mononuclear cells of patients with advanced heavily pre-treated adenocarcinoma of the stomach and gastro-esophageal junction. Cancer Immunol Immunother 63, 1273–1284 (2014). https://doi.org/10.1007/s00262-014-1596-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1596-x