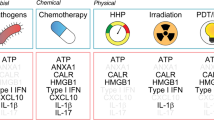
Abstract
Damage-associated molecular patterns (DAMPs), danger signal molecules expressed after injury or infection, have become recognized as prerequisite for orchestrating effective anti-tumor host response. The expression of two prototypical DAMPs, calreticulin and high-mobility group box-1 (HMGB1) protein, was examined following Photofrin™-photodynamic therapy (PDT) of Lewis lung carcinoma (LLC) cells in vitro and LLC tumors growing in syngeneic mice. Cell surface expression of calreticulin was found to be highly increased at 1 h after PDT treatment both in vitro and in vivo. Increased exposure of calreticulin was also detected on the surface of macrophages from PDT-treated LLC tumors. At the same time interval, a rise in serum HMGB1 was detected in host mice. Intracellular staining of macrophages co-incubated for 16 h with PDT-treated LLC cells revealed elevated levels of HMGB1 in these cells. The knowledge of the involvement of these DAMPs uncovers important mechanistic insights into the development of host response induced by PDT.




Similar content being viewed by others
References
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q (1998) Photodynamic therapy. J Natl Cancer Inst 90:889–905
Huang Z (2005) A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat 4:283–294
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn S, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin. doi:10.3322/caac.20114
Korbelik M (2006) PDT-associated host response and its role in the therapy outcome. Lasers Surg Med 38:500–508
Castano AP, Mroz P, Hamblin MR (2006) Photodynamic therapy and anti-tumor immunity. Nat Rev Cancer 6:535–545
Matzinger P (2002) The danger model: a renewed sense of self. Science 296:301–305
Rock KL, Kono H (2008) The inflammatory response to cell death. Annu Rev Pathol 3:99–126
Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8:279–289
Chen GY, Nuñez G (2010) Sterile inflammation: sensing and reacting to damage. Nat Re. Immunol 10:826–837
Bianchi (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–4
Miller YI, Choi S-H, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witzum JL (2011) Oxidation-specific epitopes are danger-associated molecular patterns by pattern recognition receptors of innate immunity. Circ Res 108:235–248
Srikrishna G, Freeze HH (2009) Endogenous damage-associated pattern molecules at the crossroads of inflammation and cancer. Neoplasia 11:615–628
Garg AD, Nowis D, Golab J, Agostinis P (2010) Photodynamic therapy: illuminating the road from cell death towards anti-tumor immunity. Apoptosis 15:1050–1071
Korbelik M, Stott B, Sun J (2007) Photodynamic therapy-generated vaccines: relevance of tumour cell death expression. Br J Cancer 97:1381–1387
Cecic I, Serrano K, Gyongyossy-Issa M, Korbelik M (2005) Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy. Cancer Lett 225:215–223
Korbelik M (1993) Distribution of disulfonated and tetrasulfonated aluminium phthalocyanine between malignant and host cell populations of a murine fibrosarcoma. Photochem Photobiol B: Biol 20:173–181
Stott B, Korbelik M (2007) Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol Immunother 56:649–658
Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE (2002) Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 169:3978–3986
Yang H, Hreggvidsdotir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107:11942–11947
Krosl G, Korbelik M, Dougherty GJ (1995) Induction of immune cell infiltration into murine SCCVII tumor by Photofrin-based photodynamic therapy. Br J Cancer 71:549–555
Krause KH, Michalak M (1997) Calreticulin. Cell 88:439–443
Gao B, Adhikari R, Howarth M, Nakamura K, Gold MC, Hill AB, Knee R, Michalak M, Elliott T (2002) Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity 16:99–109
Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P (2010) Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim Biophys Acta 1805:53–71
Tarr JM, Young PJ, Morse R, Shaw DJ, Haigh R, Petrov PG, Johnson SJ, Winyard PG, Eggleton P (2010) A mechanism of release of calreticulin from cells during apoptosis. J Mol Biol 401:799–812
Jeffery E, Peters LR, Raghavan M (2011) The polypeptide binding conformation of calreticulin facilitates its cell-surface expression under conditions of endoplasmic reticulum stress. J Biol Chem 286:2402–2415
Gardai SJ, Bratton DL, Ogden CA (2006) Henson PM (2006) Recognition ligands on apoptotic cells: a perspective. J Leukoc Biol 79:896–903
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini J-L, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61
Martins I, Kepp O, Galluzzi L, Senovilla L, Schlemer F, Adjemian S, Menger L, Michaud M, Zitvagel L, Kroemer G (2010) Surface-exposed calreticulin in the interaction between dying cells and phagocytes. Ann N Y Acad Sci 1209:77–82
Peng R-Q, Chen Y-B, Ding Y, Zhang R, Zhang X, Yu X-J, Zhou Z-W, Zeng Y-X, Zhang X-S (2010) Expression of calreticulin is associated with infiltration of T-cells in stage IIIB colon cancer. World J Gastroenterol 16:2428–2434
Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg P-A, Michalak M, Henson PM (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321–334
Thomas JO, Travers AA (2001) HMGB1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem Sci 26:167–174
Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Touminen RK, Lepäntalo M, Carpén O, Parkkinen J, Rauvala H (2004) Regulation of monocytes migration by amphoterin (HMGB1). Blood 104:1174–1182
Scaffidi P, T. Misteli T, Bianchi ME (2002) Release of chromatin protein HGMB1 by necrotic cells triggers inflammation. Nature 418:191–195
Yang H, Wang H, Czura CJ, Tracey KJ (2005) The cytokine activity of HMGB1. J Leukoc Biol 78:1–8
Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, Lotze MT (2010) Endogenous HMGB1 regulates autophagy. J Cell Biol 190:881–892
Semina C, Angelini G, Poggi A, Rubartelli A (2005) NK/iDC interaction results in IL-18 secretion by CD11cCD45RBhigh DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 106:609–616
Avalos AM, Kiefer K, Tian J, Christensen S, Shlomchick M, Coyle AJ, Marshak-Rothstein A (2010) RAGE-independent autoreactive B cell activation in response to chromatin and HMGB1/DNA immune complexes. Autoimmunity 43:103–110
Tang D, Kang R, Zeh HJ, Lotze MT (2011) High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 14:1315–1335
Liu QY, Yao Y-M, Yan Y-H, Dong N, Sheng Z-Y (2011) High mobility group box 1 protein suppresses T cell-mediated immunity via CD11clowCD45RBhigh dendritic cell differentiation. Cytokine (in press)
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ (in press)
El Gazzar M (2007) HMGB1 modulates inflammatory responses in LPS-activated macrophages. Inflamm Res 56:162–167
Acknowledgments
Denise McDougal provided expert assistance in flow cytometry. Axcan Pharma Inc. has provided Photofrin™ for this study.
Conflict of interest
The authors declare to have no conflict of interest in any form with respect to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korbelik, M., Zhang, W. & Merchant, S. Involvement of damage-associated molecular patterns in tumor response to photodynamic therapy: surface expression of calreticulin and high-mobility group box-1 release. Cancer Immunol Immunother 60, 1431–1437 (2011). https://doi.org/10.1007/s00262-011-1047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1047-x