Abstract
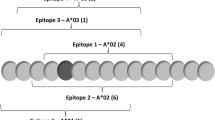
Survivin is a tumor-associated antigen with significant potential as a cancer vaccine target. We have identified a survivin peptide mimic containing human MHC class I epitopes and a potential class II ligand that induces a potent antitumor response in C57BL/6 mice with GL261 cerebral gliomas. This peptide is able to elicit both CD8+ CTL and T helper cell responses in C57BL/6 mice. The corresponding region of the human survivin molecule represented by peptide SVN53-67 is 100% homologous to the murine protein, but SVN53-67 is weakly immunogenic in man. We evaluated several amino acid substitutions in putative human MHC I anchor positions in SVN53-67 to identify potential peptide mimics that could provide an enhanced antitumor immune response against human glioma and primary central nervous system lymphoma (PCNSL) cells in culture. We evaluated survivin peptides with predicted binding to human HLA-A*0201 antigen using peptide-loaded dendritic cells from PBMC of patients with these malignancies. One alteration (M57) led to binding to HLA-A*0201 with significantly higher affinity. We compared the ability of autologous dendritic cells loaded with SVN53-67 peptide and SVN53-67/M57 in CTL assays against allomatched and autologous, survivin-expressing, human malignant glioma and PCNSL cells. Both SVN53-67 and SVN53-67/M57 produced CTL-mediated killing of malignant target cells; however, SVN53-67/M57 was significantly more effective than SVN53-67. Thus, SVN53-67/M57 may act as a peptide mimic to induce an enhanced antitumor CTL response in tumor patients. The use of SVN53-67/M57 as a cancer vaccine might have application for cancer vaccine therapy.







Similar content being viewed by others
References
Conway EM, Pollefeyt S, Cornelissen J, DeBaere I, Steiner-Mosonyi M, Ong K, Baens M, Collen D, Schuh AC (2000) Three differentially expressed survivin cDNA variants encode proteins with distinct antiapoptotic functions. Blood 95:1435–1442
Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, Ohira M, Hashizume K, Kobayashi H, Kaneko Y, Nakagawara A (2000) High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene 19:617–623
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40:1117–1123
Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC (1998) Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol 152:43–49
Ciesielski MJ, Apfel L, Barone TA, Castro CA, Weiss TC, Fenstermaker RA (2006) Antitumor effects of a xenogeneic survivin bone marrow derived dendritic cell vaccine against murine GL261 gliomas. Cancer Immunol Immunother 55:1491–1503
Chakravarti A, Noll E, Black PM et al (2002) Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol 20:1063–1068
Kajiwara Y, Yamasaki F, Hama S et al (2003) Expression of survivin in astrocytic tumors: correlation with malignant grade and prognosis. Cancer 97:1077–1083
Rohayem J, Diestelkoetter P, Weigle B et al (2000) Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res 60:1815–1817
Ciesielski MJ, Kozbor D, Castanaro CA, Barone TA, Fenstermaker RA (2008) Therapeutic effect of a T helper cell supported CTL response induced by a survivin peptide vaccine against murine cerebral glioma. Cancer Immunol Immunother 57:1827–1835
Seshadri M, Ciesielski M (2009) MRI-based characterization of vascular disruption by 5, 6-dimethylxanthenone-4-acetic acid in gliomas. J Cereb Blood Flow Metab 29:1373–1382
Rammensee HG, Bachmann J, Emmerich NN, Bachor OA, Stevanovic S (1999) SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213–219
Dionne SO et al (2003) Functional characterization of CTL against gp100 altered peptide ligands. Cancer Immunol Immunother 52:199–206
Krajewska M, Krajewski S, Banares S et al (2003) Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res 9:4914–4925
Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I (2002) Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett 184:105–116
Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I (2002) Expression of survivin is associated with malignant potential in epithelial ovarian carcinoma. Int J Mol Med 10:211–216
Yamashita S, Masuda Y, Kurizaki T et al (2007) Survivin expression predicts early recurrence in early-stage breast cancer. Anticancer Res 27:2803–2808
Andersen MH, Svane IM, Becker JC, Straten PT (2007) The universal character of the tumor-associated antigen survivin. Clin Cancer Res 13:5991–5994
Oto OA, Paydas S, Tanriverdi K, Seydaoglu G, Yavuz S, Disel U (2007) Survivin and EPR-1 expression in acute leukemias: prognostic significance and review of the literature. Leuk Res 31:1495–1501
Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, thor Straten P (2001) Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res 61:5964–5968
Hadrup SR, Gehl J, Sorensen RB, Geertsen PF, Straten PT, Andersen MH (2006) Persistence of survivin specific T cells for seven years in a melanoma patient during complete remission. Cancer Biol Ther 5:480–482
Altieri DC (2003) Validating survivin as a cancer therapeutic target. Nat Rev Cancer 3:46–54
Overwijk WW, Restifo NP (2000) Autoimmunity and the immunotherapy of cancer: targeting the “self” to destroy the “other”. Crit Rev Immunol 20:433–450
Lohr J, Knoechel B, Nagabhushanam V, Abbas AK (2005) T-cell tolerance and autoimmunity to systemic and tissue-restricted self-antigens. Immunol Rev 204:116–127
Fikes JD, Sette A (2003) Design of multi-epitope, analogue-based cancer vaccines. Expert Opin Biol Ther 3:985–993
Guevara-Patino JA, Turk MJ, Wolchok JD, Houghton AN (2003) Immunity to cancer through immune recognition of altered self: studies with melanoma. Adv Cancer Res 90:157–177
Trojan A, Witzens M, Schultze JL et al (2001) Generation of cytotoxic T lymphocytes against native and altered peptides of human leukocyte antigen-A*0201 restricted epitopes from the human epithelial cell adhesion molecule. Cancer Res 61:4761–4765
Keogh E, Fikes J, Southwood S, Celis E, Chesnut R, Sette A (2001) Identification of new epitopes from four different tumor-associated antigens: recognition of naturally processed epitopes correlates with HLA-A*0201-binding affinity. J Immunol 167:787–796
Valmori D, Fonteneau JF, Lizana CM et al (1998) Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol 160:1750–1758
Parkhurst MR, Salgaller ML, Southwood S et al (1996) Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol 157:2539–2548
Evavold BD, Sloan-Lancaster J, Allen PM (1993) Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today 14:602–609
Loftus DJ, Squarcina P, Nielsen MB et al (1998) Peptides derived from self-proteins as partial agonists and antagonists of human CD8+ T-cell clones reactive to melanoma/melanocyte epitope MART1(27-35). Cancer Res 58:2433–2439
Tynan FE, Burrows SR, Buckle AM et al (2005) T cell receptor recognition of a ‘super-bulged’ major histocompatibility complex class I-bound peptide. Nat Immunol 6:1114–1122
Tynan FE, Reid HH, Kjer-Nielsen L et al (2007) A T cell receptor flattens a bulged antigenic peptide presented by a major histocompatibility complex class I molecule. Nat Immunol 8:268–276
Pardoll DM (1999) Inducing autoimmune disease to treat cancer. Proc Natl Acad Sci USA 96:5340–5342
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H (1998) The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 188:2357–2368
Kim W, Liau LM (2010) Dendritic cell vaccines for brain tumors. Neurosurg Clin N Am 21:139–157
Wheeler CJ, Black KL (2009) DCVax-Brain and DC vaccines in the treatment of GBM. Expert Opin Investig Drugs 18:509–519
Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE 2nd, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, Friedman HS, Bigner DD (2009) An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther 8:2773–2779
Heimberger AB, Sampson JH (2009) The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther 9:1087–1098
Acknowledgments
This work was supported by NIH 5R21 NS049309-02 (RAF), NIH 5P30 CA16056-29, the Linda Scime Fund of the Roswell Park Alliance Foundation, and the Glioblastoma Multiforme Grant awarded to MJC by the National Brain Tumor Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciesielski, M.J., Ahluwalia, M.S., Munich, S.A. et al. Antitumor cytotoxic T-cell response induced by a survivin peptide mimic. Cancer Immunol Immunother 59, 1211–1221 (2010). https://doi.org/10.1007/s00262-010-0845-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0845-x