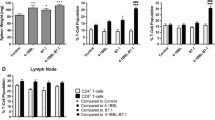
Abstract
Survivin is a member of the inhibitor of apoptosis protein family. Gliomas and many other tumors express survivin at high levels; whereas, normal fully differentiated cells generally do not. Therefore, survivin represents a tumor-specific target for cancer vaccine therapy. It has been shown that it is possible to produce a MHC-I-restricted cellular immunologic response to survivin vaccines. To study differences in immunogenicity between murine and human survivin proteins, we vaccinated C57BL/6 mice with bone marrow dendritic cells (BMDC) transfected with expression vectors containing the murine and human survivin genes. Mice vaccinated with BMDCs expressing a truncated human survivin protein developed cytotoxic T lymphocyte to subcutaneous GL261 glioma cells and exhibited prolonged tumor-free survival compared to mice vaccinated with BMDCs transfected with vector alone (P<0.01). While mice challenged with intracerebral GL261 cells had increased survival, no cures were observed. In contrast, vaccinated mice that fully resisted subcutaneous tumor challenge were rendered resistant to intracerebral GL261 re-challenge. BMDCs transfected with the full-length human survivin molecule were significantly more effective at prolonging survival than BMDCs expressing the full-length murine survivin gene (P=0.0175). Therefore, xenogeneic differences between human and murine sequences might be exploited to develop more immunogenic tumor vaccines.








Similar content being viewed by others
References
Moscatello DK, Ramirez G, Wong AJ (1997) A naturally occurring mutant epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res 57:1419–1424
Heimberger AB, Crotty LE, Archer GE et al (2003) Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res 9:4247–4254
Ciesielski MJ, Kazim L, Barth RF, Fenstermaker RA (2005) Cellular antitumor immune response to a branched lysine multiple antigenic peptide containing epitopes of a common tumor-specific antigen in a rat glioma model. Cancer Immunol Immunother 54:107–119
Prins RM, Odesa SK, Liau LM (2003) Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model. Cancer Res 63:8487–8491
Adida C, Crotty PL, McGrath J et al (1998) Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol 152:43–49
Kajiwara Y, Yamasaki F, Hama S et al (2003) Expression of survivin in astrocytic tumors. Cancer 97:1077–1083
Sasaki T, Lopes MB, Hankins GR, Helm GA (2002) Expression of survivin an inhibitor of apoptosis protein in tumors of the nervous system. Acta Neuropathol (Berl) 104:105–109
Chakravarti A, Noll E, Black P et al (2002) Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol 20:1063–1068
Islam A, Kageyama H, Takada N et al (2000) A high expression of survivin mapped to 17q25 is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene 19:617–623
Satoh K, Kaneko K, Hirota M et al (2001) T expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer 92:271–278
Altieri DC (2003) Validating survivin as a cancer therapeutic target. Nat Rev Cancer 3:46–54
Overwijk WW, Tsung A, Irvine KR et al (1998) gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive tumoricidal T cells using high-affinity altered peptide ligand. J Exp Med 188:277–286
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:2445–2452
Steinman RM, Dhodapkar M (2001) Active immunization against cancer with dendritic cells: the near future. Int J Cancer 4:459–473
Patel AK, Boyd PN (1995) An improved assay for antibody dependent cellular cytotoxicity based on time resolved fluorometry. J Immunol Methods 184(1):29–38
Liau LM, Black KL, Martin NA et al (2000) Treatment of a glioblastoma patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Neurosurg Focus 9:8
Yu JS, Wheeler CJ, Zeltzer PM et al (2001) Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61:842–847
Yu JS, Liu G, Ying H et al (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific cytotoxic T-cells in patients with malignant glioma. Cancer Res 64:4973–4999
Kikuchi T, Akasaki Y, Irie M et al (2001) Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother 50:337–344
Zhao J, Tenev T, Martins LM et al (2000) The ubiquitin-proteosome pathway regulates survivin degradation in a cell-cycle dependent manner. J Cell Sci 113:4363–4371
Li F, Ambrosini G, Chu EY et al (1998) Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 396:580–584
Fortugno P, Wall NR, Giodini A et al (2002) Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci 115:575–585
Tamm I, Wang Y, Sausville E et al (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95) Bax caspases and anticancer drugs. Cancer Res 58:5215–5220
Conway EM, Pollefeyt S, Cornelissen J et al (2000) Three differentially expressed survivin cDNA variants encode proteins with distinct antiapoptotic functions. Blood 95:1435–1442
Shin S, Sung BJ, Cho Y et al (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40:1117–1123
Fududa S, Mantel CR, Pelus LM (2004) Survivin regulates hematopoietic progenitor cell proliferation through p21WAF1/Cip1 dependent and independent pathways. Blood 103:120–127
Suzuki A, Hayashida M, Ito T et al (2000) Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16INK4a and Cdk2/Cyclin E complex activation. Oncogene 19:3225–3334
Grossman D, Kim PJ, Schechner JS, Altieri DC (2001) Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci USA 98:635–640
Mesri M, Wall NR, Li J et al (2001) Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest 108:981–990
Xiang R, Mizutani N, Luo Y et al (2005) A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res 65:553–561
Zeis M, Siegel S, Wagner A et al (2003) A generation of cytotoxic responses in mice and human individually against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol 170:5391–5397
Andersen MH, Pedersen LO, Becker JC, Straten P (2001) Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res 61:869–872
Schmitz M, Diestelkoetter P, Weigle B et al (2000) Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res 60:4845–4849
Yamanaka R, Yajima N, Abe T et al (2003) Dendritic cell-based glioma immunotherapy. Int J Oncol 23:5–15
Yang L, Ng KY, Lillehei KO (2003) Cell-mediated immunotherapy: a new approach to the treatment of malignant glioma. Cancer Control 10:138–147
Ehtesham M, Black KL, Yu JS (2004) Recent progress in immunotherapy for malignant glioma: treatment strategies and results from clinical trials. Cancer Control 11:192–207
Dunn GP, Bruce AT, Ikeda H (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991–998
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:2445–2452
Steinman RM, Dhodapkar M (2001) Active immunization against cancer with dendritic cells: the near future. Int J Cancer 4:459–473
Ni HT, Spellman SR, Jean WC et al (2001) Immunization with dendritic cells pulsed with tumor extract increases survival of mice bearing intracranial gliomas. J Neurooncol 51:1–9
Aoki H, Mizuno M, Natsume A et al (2001) Dendritic cells pulsed with tumor extract-cationic liposome complex increase the induction of cytotoxic T lymphocytes in mouse brain tumor. Cancer Immunol Immunother 50:463–468
Gordan JD, Vonderheide RH (2002) Universal tumor antigens as targets for immunotherapy. Cytotherapy 4:317–327
Altieri DC (2003) Survivin and apoptosis control. Adv Cancer Res 88:31–52
O’Connor DS, Schechner JS, Adida C et al (2000) Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol 156:393–398
Altieri DC (2001) The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med 7:542–547
Andersen MH, Pedersen LO, Capeller B et al (2001) Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res 61:5964–5968
Reker S, Meier A, Holten-Andersen L, Svane IM, Becker JC, thor Straten P, Andersen MH (2004) Identification of novel survivin-derived CTL epitopes. Cancer Biol Ther 3(2):173–179
Fenstermaker RA, Ciesielski MJ (2004) Immunotherapeutic strategies for malignant gliomas. Cancer Control 11:181–191
Hawkins WG, Gold JS, Dyall R (2000) Immunization with DNA coding for gp100 results in CD4 T-cell independent antitumor immunity. Surgery 128:273–280
Luo W, Hsu JC, Tsao CY, Ko E, Wang X, Ferrone S (2005) Differential immunogenicity of two peptides isolated by high molecular weight-melanoma-associated antigen-specific monoclonal antibodies with different affinities. J Immunol 174:7104–7110
Kieber-Emmons TB, Monzavi-Karbassi B, Wang P et al (2000) Cutting edge: DNA immunization with minigenes of carbohydrate mimotopes induce functional anti-carbohydrate antibody response. J Immunol 165:623
Cunto-Amesty GP, Luo B, Monzavi-Karbassi A et al (2003) Peptide mimotopes as prototypic templates of broad-spectrum surrogates of carbohydrate antigens. Cell Mol Biol 49:245
Chapman PBD, Wu G, Ragupathi S et al (2004) Sequential immunization of melanoma patients with GD3 ganglioside vaccine and anti-idiotypic monoclonal antibody that mimics GD3 ganglioside. Clin Cancer Res 10:4717
Acknowledgements
This work was supported by NIH 5R21NS049309–02, NIH 5P30CA16056-29, the Roswell Park Alliance Foundation and the Linda Scime Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciesielski, M.J., Apfel, L., Barone, T.A. et al. Antitumor effects of a xenogeneic survivin bone marrow derived dendritic cell vaccine against murine GL261 gliomas. Cancer Immunol Immunother 55, 1491–1503 (2006). https://doi.org/10.1007/s00262-006-0138-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-006-0138-6