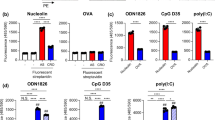
Abstract
Due to the inherent lack of immunogenicity of peptides, it is generally recognized that the strong inflammatory signals that are required to elicit specific responses against peptide-based therapeutic tumor vaccines may not be provided by the standard/conventional vaccine adjuvants. In this study, we have demonstrated dsRNA in the form of synthetic pI:C as a potent adjuvant to enhance the specific anti-tumor immune responses against a peptide-based vaccine. When complexed with an MHC I-restricted minimal peptide epitope derived from the HPV 16 E7 protein, the resulting pI:C/E749–57 molecular complex induced strong E749–57-specific CTL responses that caused significant regressions of model human cervical cancer tumors pre-established in mice. In addition, although the proportion of DCs in tumor-bearing mice was significantly decreased when compared to that in naïve mice, immunization with pI:C/E749–57 restored the proportion of DCs in tumor-bearing mice. Double-stranded RNA may hold a great potential as an adjuvant to induce cellular immune responses for tumor immunotherapy.










Similar content being viewed by others
Abbreviations
- CTL:
-
Cytotoxic T lymphocyte
- DC:
-
Dendritic cells
- LN:
-
Lymph node
- HPV:
-
Human papillomavirus
- TLR:
-
Toll-like receptor
- PAMP:
-
Pathogen-associated molecular pattern
- MHC:
-
Major histocompatibility complex
- pDC:
-
Plasmocytoid DC
- mDC:
-
Myeloid DC
- NK:
-
Natural killer
- pI:C or Poly(I:C):
-
Polyinosine-polycytidylic acid
- CFSE:
-
5-(and-6-)-carboxylfluorescein diacetate, succinimidayl ester
- s.c.:
-
Subcutaneous
- HLA:
-
Human lymphocyte antigen
- IFN:
-
Interferon
- TAA:
-
Tumor-associated antigens
- TSA:
-
Tumor-specific antigens
- IFA:
-
Incomplete Freund’s adjuvant
References
Buteau C, Markovic SN, Celis E (2002) Challenges in the development of effective peptide vaccines for cancer. Mayo Clin Proc 77(4):339–349
Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM (1996) CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA 93:2879–2883
Davila E, Kennedy R, Celis E (2003) Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res 63(12):3281–3288
Davila E, Celis E (2000) Repeated administration of cytosine-phosphorothiolated guanine-containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J Immunol 165(1):539–547
Miconnet I, Koenig S, Speiser D et al. (2002) CpG are efficient adjuvants for specific CTL induction against tumor antigen-derived peptide. J Immunol 168(3):1212–1218
Speiser DE, Lienard D, Rufer N et al. (2005) Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 115(3):739–746
Kadowaki N, Ho S, Antonenko S et al. (2001) Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194:863–869
Ulevitch RJ (2004) Therapeutics targeting the innate immune system. Nat Rev Immunol 4(7):512–520
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738
Herman R, Baron S (1971) Immunologic-mediated protection of Trypanosoma congolense-infected mice by polyribonucleotides. J Protozool 18(4):661–666
Park JH, Baron S (1968) Herpetic keratoconjunctivitis: therapy with synthetic double-stranded RNA. Science 162(855):811–813
Partidos CD, Hoebeke J, Moreau E et al. (2005) The binding affinity of double-stranded RNA motifs to HIV-1 Tat protein affects transactivation and the neutralizing capacity of anti-Tat antibodies elicited after intranasal immunization. Eur J Immunol 35(5):1521–1529
Ichinohe T, Watanabe I, Ito S et al. (2005) Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol 79(5):2910–2919
Fujimoto C, Nakagawa Y, Ohara K, Takahashi H (2004) Polyriboinosinic polyribocytidylic acid [poly(I:C)]/TLR3 signaling allows class I processing of exogenous protein and induction of HIV-specific CD8+ cytotoxic T lymphocytes. Int Immunol 16(1):55–63
Meier WA, Husmann RJ, Schnitzlein WM, Osorio FA, Lunney JK, Zuckermann FA (2004) Cytokines and synthetic double-stranded RNA augment the T helper 1 immune response of swine to porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 102(3):299–314
Verdijk RM, Mutis T, Esendam B et al. (1999) Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol 163(1):57–61
Schmidt KN, Leung B, Kwong M et al. (2004) APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol 172(1):138–143
Sivori S, Falco M, Della Chiesa M et al. (2004) CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA 101(27):10116–10121
Gelman AE, Zhang J, Choi Y, Turka LA (2004) Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol 172(10):6065–6073
Salem ML, Kadima AN, Cole DJ, Gillanders WE (2005) Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother 28(3):220–228
Schulz O, Diebold SS, Chen M et al. (2005) Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433(7028):887–892
Franco EL, Schlecht NF, Saslow D (2003) The epidemiology of cervical cancer. Cancer J 9(5):348–359
zur Hausen H, de Villiers EM (1994) Human papillomaviruses. Annu Rev Microbiol 48:427–447
Munoz N, Bosch FX, de Sanjose S et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348(6):518–527
Scheffner M, Huibregtse JM, Vierstra RD, Howley PM (1993) The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495–505
Munger K, Scheffner M, Huibregtse JM, Howley PM (1992) Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv 12:197–217
Borysiewicz LK, Fiander A, Nimako M et al. (1996) A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 347:1523–1527
Kaufmann AM, Stern PL, Rankin EM et al. (2002) Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin Cancer Res 8:3676–3685
Ferrara A, Nonn M, Sehr P et al. (2003) Dendritic cell-based tumor vaccine for cervical cancer II: results of a clinical pilot study in 15 individual patients. J Cancer Res Clin Oncol 129:521–530
Muderspach L, Wilczynski S, Roman L et al. (2000) A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res 6:3406–3416
Steller MA, Gurski KJ, Murakami M et al. (1998) Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res 4(9):2103–2109
van Driel WJ, Ressing ME, Kenter GG et al. (1999) Vaccination with HPV16 peptides of patients with advanced cervical carcinoma: clinical evaluation of a phase I-II trial. Eur J Cancer 35(6):946–952
Lin CW, Lee JY, Tsao YP, Shen CP, Lai HC, Chen SL (2002) Oral vaccination with recombinant Listeria monocytogenes expressing human papillomavirus type 16 E7 can cause tumor growth in mice to regress. Int J Cancer 102(6):629–637
Hwu P, Yang JC, Cowherd R et al. (1995) In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer Res 55(15):3369–3373
Cui Z, Han SJ, Huang L (2004) Coating of mannan on LPD particles containing HPV E7 peptide significantly enhances immunity against HPV-positive tumor. Pharm Res 21:1018–1025
Cui Z, Huang L (2005) Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: Therapeutic effect against cervical cancer. Cancer Immunol Immunother 54(12):1180–1190
Dileo J, Banerjee R, Whitmore M, Nayak JV, Falo LD Jr, Huang L (2003) Lipid-protamine-DNA-mediated antigen delivery to antigen-presenting cells results in enhanced anti-tumor immune responses. Mol Ther 7:640–648
Curtsinger JM, Johnson CM, Mescher MF (2003) CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol 171(10):5165–5171
den Boer AT, van Mierlo GJ, Fransen MF, Melief CJ, Offringa R, Toes RE (2004) The tumoricidal activity of memory CD8+ T cells is hampered by persistent systemic antigen, but full functional capacity is regained in an antigen-free environment. J Immunol 172(10):6074–6079
Toes RE, Offringa R, Blom RJ, Melief CJ, Kast WM (1996) Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci USA 93(15):7855–7860
Hoerr I, Obst R, Rammensee HG, Jung G (2000) In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol 30(1):1–7
Blattman JN, Greenberg PD (2004) Cancer immunotherapy: a treatment for the masses. Science 305(5681):200–205
Berzofsky JA, Ahlers JD, Janik J et al. (2004) Progress on new vaccine strategies against chronic viral infections. J Clin Invest 114(4):450–462
Walboomers JM, Jacobs MV, Manos MM et al. (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189(1):12–19
Ong MK, Glantz SA (2004) Cardiovascular health and economic effects of smoke-free workplaces. Am J Med 117(1):32–38
Franceschi S (2005) The IARC commitment to cancer prevention: the example of papillomavirus and cervical cancer. Recent Results Cancer Res 166:277–297
Kadish AS, Timmins P, Wang Y et al. (2002) Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol Biomarkers Prev 11:483–488
Hirabayashi K, Yano J, Inoue T et al. (1999) Inhibition of cancer cell growth by polyinosinic-polycytidylic acid/cationic liposome complex: a new biological activity. Cancer Res 59(17):4325–4333
Hemmi H, Takeuchi O, Kawai T et al. (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408(6813):740–745
Krieg AM, Yi AK, Matson S et al. (1995) CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374(6522):546–549
Jacobs BL, Langland JO (1996) When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219(2):339–349
Lindh HF, Lindsay HL, Mayberry BR, Forbes M (1969) Polyinosinic-cytidylic acid complex (poly I:C) and viral infections in mice. Proc Soc Exp Biol Med 132(1):83–87
Tabeta K, Georgel P, Janssen E et al. (2004) Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA 101(10):3516–3521
Yang L, Carbone DP (2004) Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res 92:13–27
Almand B, Resser JR, Lindman B et al. (2000) Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 6(5):1755–1766
Gabrilovich DI, Chen HL, Girgis KR et al (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2(10):1096–1103
Morelli AE, Larregina AT, Ganster RW et al. (2000) Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol 74(20):9617–9628
Muraille E, De Trez C, Pajak B, Torentera FA, De Baetselier P, Leo O, Carlier Y (2003) Amastigote load and cell surface phenotype of infected cells from lesions and lymph nodes of susceptible and resistant mice infected with Leishmania major. Infect Immun 71(5):2704–2715
Coates PT, Barratt-Boyes SM, Zhang L et al. (2003) Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood 102(7):2513–2521
Fischer HG, Bonifas U, Reichmann G (2000) Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol 164(9):4826–4834
Nishi T, Okazaki K, Kawasaki K et al. (2003) Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect Immun 71(4):2153–2162
Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC (2004) Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod 70(4):1018–1023
Turner BC, Hemmila EM, Beauchemin N, Holmes KV (2004) Receptor-dependent coronavirus infection of dendritic cells. J Virol 78(10):5486–5490
Schiavoni G, Mattei F, Sestili P et al. (2002) ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med 196(11):1415–1425
Kruger T, Benke D, Eitner F et al. (2004) Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 15(3):613–621
Kadowaki N, Antonenko S, Liu YJ (2001) Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c- type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J Immunol 166(4):2291–2295
Strayer DR, Carter WA, Brodsky I et al. (1994) A controlled clinical trial with a specifically configured RNA drug, poly(I).poly(C12U), in chronic fatigue syndrome. Clin Infect Dis 18(Suppl 1):S88–95
Adams M, Navabi H, Jasani B et al. (2003) Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R). Vaccine 21(7–8):787–790
Fernando GJ, Khammanivong V, Leggatt GR, Liu WJ, Frazer IH (2002) The number of long-lasting functional memory CD8+ T cells generated depends on the nature of the initial nonspecific stimulation. Eur J Immunol 32(6):1541–1549
Acknowledgement
Flow cytometry analyses were completed in the Flow Cytometry and Cell Sorting Facilities in the Environmental Health Science Center at the Oregon State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Z., Qiu, F. Synthetic double-stranded RNA poly(I:C) as a potent peptide vaccine adjuvant: therapeutic activity against human cervical cancer in a rodent model. Cancer Immunol Immunother 55, 1267–1279 (2006). https://doi.org/10.1007/s00262-005-0114-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-005-0114-6