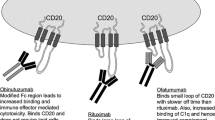
Abstract
Cure of patients with chronic lymphocytic leukemia (CLL) has been an elusive goal. The recent availability of active monoclonal antibodies has rekindled enthusiasm for new and innovative therapeutic approaches. Alemtuzumab, induces responses in about a third of patients with relapsed or refractory CLL following therapy with fludarabine and an alkylating agent. Whereas, rituximab has limited activity in previously treated patients, response rates of 50–70% have been reported in those without prior therapy. Recent data on combinations with rituximab and chemotherapy have shown promise for improving patient outcome. Newer antibodies in development include the primatized monoclonal antibody lumiliximab (IDEC-152), directed against CD23. Other biological approaches include the use of antisense oligonucleotides, proapoptic small molecules, and vaccines directed against the malignant B cells. The rational development of combinations of these promising approaches may eliminate the need for chemotherapy, leading to safer and more effective approaches for patients with CLL.
Similar content being viewed by others
References
Leporrier M, Chevret S, Cazin B et’al (2001) Randomized comparison of fludarabine, CAP, and ChOP, in 938 previously treated stage B and C-chronic lymphocytic leukemia. Blood 98:2319–2325
Rai KR, Peterson BL, Kolitz J et’al (2000) Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. New Engl J Med 343:1750–1757
Ehrlich P (1900) The Croonian lecture: “on immunity with special reference to cell life”. Proc Royal Soc 66:424–448
Nadler LM, Ritz J, Hardy R et’al (1981) A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest 67:134–140
Nadler LM, Stashenko P, Hardy R et’al (1980) Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res 40:3147–3154
Miller RA, Maloney DG, Warnke R, Levy R (1982) Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. New Engl J Med 306:517–522
Kohler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497
Dillman RO, Shawler DL, Dillman JB, Royston I (1984) Therapy of chronic lymphocytic leukemia and cutaneous T-cell lymphoma with T101 monoclonal antibody. J Clin Oncol 2:881–891
Hertler AA, Schlossman DM, Borowitz MJ et’al (1988) A phase I study of T101-ricin A chain immunotoxin in refractory chronic lymphocytic leukemia. J Biol Resp Mod 7:97–113
Zimmer AM, Kaplan EH, Kazikiewicz JM et’al (1988) Pharmacokinetics of I-131 T101 monoclonal antibody in patients with chronic lymphocytic leukemia. Antibody Immunoconjugates Radiopharm 1:291–303
Laurent G, Pris J, Farcet J-P et’al (1986) Effects of therapy with T101 ricin A-chain immunotoxin in two leukemia patients. Blood 67:1680–1687
Capel PJA, Preijers FWMB, Allebes WA, Haanen C (1985) Treatment of chronic lymphocytic leukaemia with monoclonal anti-idiotype antibody. Neth J Med 28:112–118
Allebes WA, Preijers FWMB, Haanen C, Capel PJA (1988) The development of non-responsiveness to immunotherapy with monoclonal anti-idiotypic antibodies in a patient with B-CLL. Br J Haematol 70:295–300
Hale G, Xia M-Q, Tighe HP et’al (1990) The CAMPATH-1 antigen (CDw52). Tissue Antigens 35:118–127
Ginaldi L, De martinis M, Matutes E et’al (1998) Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to CAMPATH-1H. Leuk Res 22:185–191
Dyer MJS, Hale G, Hayhoe FGJ, Waldmann H (1989) Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: Influence of antibody isotype. Blood 73:1431–1439
Dyer MJ (1999) The role of CAMPATH-1 antibodies in the treatment of lymphoid malignancies. Semin Oncol 26:52–57
Dyer MJ, Hale G, Marcus RE, Waldmann H (1990) Remission induction in patients with lymphoid malignancies using unconjugated CAMPATH-1 monoclonal antibodies. Leuk Lymph 2:179–190
Österborg A, Dyer MJS, Bunjes D et’al (1997) Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. J Clin Oncol 15:1567–1574
Bowen AL, Zomas A, Emmett E et’al (1997) Subcutaneous CAMPATH-1H in fludarabine-resistant/relapsed chronic lymphocytic and B-prolymphocytic leukaemia. Br J Haematol 96:617–619
Rawstron AC, Davies FE, Evans P et’al (1997) CAMPATH1H therapy for patients with refractory chronic lymphocytic leukemia (CLL). Blood 90:529a (abstr 2356)
Keating MJ, Flinn I, Jain V et’al (2002) Therapeutic role of alemtuzumab (CAMPATH-1H) in patients who have failed fludarabine: results of a large international study. Blood 99:3554–3561
Österborg A, Fassas AS, Anagnostopoulos A et’al (1996) Humanized CD52 monoclonal antibody Campath-1H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol 93:151–153
McCune SL, Gockerman JP, Moore JO et’al (2002) Alemtuzumab in relapsed or refractory chronic lymphocytic leukemia and prolymphocytic leukemia. Leuk Lymph 43:1007–1011
Lundin J, Kimby E, Björkholm M et’al (2002) Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (CAMPATH-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 100:768–773
Rai KR, Coutré S, Rizzieri D et’al (2001) Efficacy and safety of alemtuzumab (CAMPATH-1H) in refractory B-CLL patients treated on a compassionate basis. Blood 98:365a (abstr 1538)
Stilgenbauer S, Döhner H (2002) Campath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 mutation and resistance to chemotherapy. New Engl J Med 347:452–452
Perkins JG, Flynn JM, Howard RS, Byrd JC (2002) Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: implications for clinical trials in this patient population. Cancer 94:2033–2039
Hale G, Rebello P, Breitman LR et’al (2004) Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood 104:948–955
Stilgenbauer S, Winkler D, Kröber A et’al (2004) Subcutaneous Campath-1H (alemtuzumab) in fludarabine-refractory CLL: Interim analysis of the CLL2h study of the German CLL Study Group (GCLLSG). Blood 104:140a (abstr#478)
Rai KR, Byrd JC, Peterson B et’al (2003) Subcutaneous alemtuzumab following fludarabine for previously untreated patients with chronic lymphocytic leukemia (CLL): CALGB study 19901. Blood 102:676–677a (abstr#2506)
Rai KR, Byrd JC, Peterson B, Larson RA (2002) A phase II trial of fludarabine followed by alemtuzumab (CAMPATH-1H) in previously untreated chronic lymphocytic leukemia (CLL) patients with active disease: Cancer and Leukemia Group B (CALGB) Study 19901. Blood 100:205a (abstr 772)
Kennedy B, Rawstron A, Carter C et’al (2002) CAMPATH-1H and fludarabine in combination are highly active in refractory chronic lymphocytic leukemia. Blood 99:2245–2247
Elter T, Bochmann P, Schulz H et’al (2004) FluCam - a new, 4-weekly combination of fludarabine and alemtuzumab for patients with relapsed chronic lymphocytic leukemia. Blood 104:690a (abstr 2517)
Montillo M, Tedeschi A, Rossi V et’al (2004) Alemtuzumab as consolidation after a response to fludarabine is effective to purge residual disease in patients with chronic lymphocytic leukemia. Blood 104:140a (abstr#479)
Oȁ9Brien SM, Kantarjian HM, Thomas DA et’al (2003) Alemtuzumab as treatment for residual disease after chemotherapy on patients with chronic lymphocytic leukemia. Cancer 98:2657–2663
Wendtner C-M, Ritgen M, Schweighofer CD et’al (2004) Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission - experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL study group. Leukemia 18:1093–1101
Moreton P, Kennedy B, Lucas G et’al (2005) Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol epub, Feb 28
Wierda W, Faderl S, Oȁ9Brien S et’al (2004) Combined cyclophosphamide, fludarabine, alemtuzumab, and rituximab (CFAR) is active for relapsed and refractory patients with CLL. Blood 104:101a (abstr#340)
Lozanski G, Heerema NA, Flinn IW et’al (2004) Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood 103:3278–3281
Döhner H, Fischer K, Bentz M et’al (1995) p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood 85:1580–1589
Lin TS, Flinn IW, Modali R et’al (2004) FCGR3A and FCGR2A polymorphisms may not correlate with response to alemtuzumab (CAMPATH-1H) in chronic lymphocytic leukemia. Blood (in press)
Rawstron AC, Kennedy B, Moreton P et’al (2004) Early prediction of outcome and response to alemtuzumab therapy in chronic lymphocytic leukemia. Blood 103:2027–2031
McLaughlin P, Grillo-López AJ, Link BK et’al (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy of relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833
Harris NL, Jaffe ES, Diebold J et’al (1999) World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the clinical advisory committee meeting—Airlie House, Virginia. J Clin Oncol 17:3835–3849
Nguyen DT, Amess JA, Doughty H et’al (1999) IDEC-C2B8 anti-CD20 (rituximab) immunotherapy in patients with low-grade non-Hodgkinȁ9s lymphoma and lymphoproliferative disorders: evaluation of response on 48 patients. Eur J Haematol 62:76–82
Maloney DG, Grillo-López AJ, White CA et’al (1997) IDEC-C2B8 (Rituximab) anti-CD20- monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkinȁ9s lymphoma. Blood 90:2188–2195
Piro LD, White CA, Grillo-Lopez AJ et’al (1999) Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkinȁ9s lymphoma. Ann Oncol 10:655–661
Winkler U, Jensen M, Manzke O et’al (1999) Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (Rituximab, IDEC-C2B8). Blood 94:2217–2224
Foran JM, Rohatiner AZ, Cunningham D et’al (2000) European phase II study of rituximab (Chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol 18:317–324
Oȁ9Brien SM, Kantarjian H, Thomas DA et’al (2001) Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 19:2165–2170
Byrd JC, Murphy T, Howard RS et’al (2001) Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol 19:2153–2164
Huhn D, von Schilling C, Wilhelm M et’al (2001) Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood 98:1326–1331
Hainsworth JD, Lichty S, Barton JH et’al (2003) Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 21:1746–1751
Hainsworth JD, Lichty S, Burris HA III et’al (2002) Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkinȁ9s lymphoma. J Clin Oncol 20:4261–4267
Byrd JC, Peterson B, Morrison VA et’al (2003) Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 101:6–14
Byrd JC, Rai K, Peterson BL et’al (2005) The addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: An updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood 105:49–53
Keating MJ, Oȁ9Brien S, Albitar M et’al (2005) Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab (FCR) as initial therapy for chronic lymphocytic leukemia. J Clin Oncol epub ahead of print, March 14
Wierda W, Oȁ9Brien S, Wen S et’al (2005) Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol epub ahead of print, March 14
Ghazal HH (2001) Successful treatment of pure red cell aplasia (PRA) with rituxan in patients with CLL. Blood 98:219a (abstr 914)
Hegde UP, Wilson WH, White T, Cheson BD (2002) Rituximab treatment of refractory fludarabine -associated immune thrombocytopenia in chronic lymphocytic leukemia. Blood 100:2260–2262
Nabhan C, Patton D, Gordon L et’al (2004) A pilot trial of rituximab and alemtuzumab combination therapy in patients with relapsed and/or refractory chronic lymphocytic leukemia. Leuk Lymph 45:2269–2273
Faderl S, Thomas DA, Oȁ9Brien S et’al (2003) Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood 101:3413–3415
Dillman RO (1999) Infusion reactions associated with the therapeutic use of monoclonal antibodies in the treatment of malignancy. Cancer Metastasis Rev 18:465–471
Byrd JC, Waselenko JK, Maneatis T et’al (1999) Rituximab therapy in hematologic malignancy patients with circulating tumor cells: association with increased infusion-related side effects and rapid blood tumor clearance. J Clin Oncol 17:791–795
Cheson BD, Frame JN, Vena D et’al (1998) Tumor lysis syndrome: an uncommon complication of fludarabine therapy of chronic lymphocytic leukemia. J Clin Oncol 16:2313–2320
Rai KR, Oȁ9Brien S, Cunningham C et’al (2002) Genasense (Bcl-2 antisense) monotherapy in patients with relapsed or refractory chronic lymphocytic leukemia: phase I and II results. Blood 100:384a (abstr 1490)
Rai KR, Moore JO, Boyd TE et’al (2004) Phase 3 randomized trial of fludarabine/cyclophosphamide chemotherapy with or without oblimerson sodium (Bcl-2 antisense; Genasense; G3139) for patients with relapsed or refractory chronic lymphocytic leukemia (CLL). Blood:abstr 338
Chanan-Khan AA, Mavromatis B, Rai KR et’al (2004) A pilot study of Genasense (Oblimersen sodium, Bcl-2 antisense oligonucleotide), fludarabine and rituximab in previously treated and untreated subjects with chronic lymphocytic leukemia. Blood 104:abstr 4827
Pathan N, Hariharan K, Hopkins M et’al (2001) Induction of apoptosis by IDEC-152 (anti-CD23) in lymphoma cells. Blood 98:367a (abstr 1545)
Byrd J, Oȁ9Brien S, Flinn I et’al (2003) Interim results from a phase I study of lumiliximab (IDEC-152, anti-CD23 antibody) therapy for relapsed or refractory CLL. Blood 102:abstr 248
König A, Menzel T, Lynen S et’al (1997) Basic fibroblast growth factor (bFGF) upregulates the expression of bcl-2 in B cell chronic lymphocytic leukemia cell lines resulting in delaying apoptosis. Leukemia 11:258265
Langmuir VK, Cobleigh MA, Herbst RS et’al (2002) Successful long-term therapy with bevacizumab (Avastin) in solid tumors. Proc Amer Soc Clin Oncol 21:9a (abstr 32)
Figg D, Fruger EA, Price DK (2002) Inhibition of angiogenesis: treatment options for patients with metastatic prostate cancer. Inv New Drugs 20:183–194
Karp JE, Gojo I, Gocke CD et’al (2002) Timed sequential therapy (TST) of relapsed and refractory adult acute myelogenous leukemia (AML) with the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab. Blood 100:198a (abstr 744)
Cheson BD (2003) Radioimmunotherapy of non-Hodgkinȁ9s lymphomas. Blood 101(in press) (Jan 15)
Dillman RO (2002) Radiolabeled anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoma. J Clin Oncol 20:3545–3547
DeNardo GJ, Lewis JP, DeNardo SJ, Oȁ9Grady LF (1993) Effect of Lym-1 radioimmunoconjugate on refractory chronic lymphocytic leukemia. Cancer 73:1425–1432
Epstein AL, Marder RJ, Winter JN et’al (1987) Two new monoclonal antibodies, Lym-1 and Lym-2, reactive with human B-lymphocytes and derived tumors, with immunodiagnostic and immunotherapeutic potential. Cancer Res 47:830–840
Lewis JP, DeNardo GL, DeNardo SJ, Oȁ9Grady LF (1990) Impact of Lym-1 radioimmunoconjugate on refractory chronic lymphocytic leukemia (CLL). Blood 76:295a (abstr 1171)
DeNardo SJ, DeNardo GL, Oȁ9Grady LF et’al. (1988) Pilot studies of radioimmunotherapy of B cell lymphoma and leukemia using I-131 Lym-1 monoclonal antibody. Antibody Immunoconjugates Radiopharm 1:17–33
DeNardo GL, DeNardo SJ, Oȁ9Grady LF et’al (1990) Fractionated radioimmunotherapy of B-cell malignancies with 131 I-Lym-1. Cancer Res 50:1014s–1016s
Hu E, Epstein AL, Naeve GS et’al (1989) A phase Ia clinical trial of LYM-1 monoclonal antibody serotherapy in patients with refractory B cell malignancies. Hematol Oncol 7:155–166
Kaminski MS, Press OW, Lister TA et’al (1999) Iodine I131 tositumomab for patients with small lymphocytic lymphoma (SLL): overall clinical trial experience. Blood 94:abstr 386
McGreivy JS, Marshall J, Cheson BD et’al (2005) Initial results from ongoing Phase I trials of a novel pan bcl-2 family small molecule inhibitor. Proc ASCO (in press)
Krackhardt AM, Harig S, Witzens M et’al (2002) T-cell responses against chronic lymphocytic leukemia cells: implications for immunotherapy. Blood 100:167–173
Kater AP, Remmerswaal EBM, Nolte MA et’al (2004) Autologous cytomegalovirus-specific T cells as effector cells in immunotherapy of B cell chronic lymphocytic leukaemia. Br J Haematol 126:512–516
Gitelson E, Hammond C, Mena J et’al (2003) Chronic lymphocytic leukemia-reactive T cells during disease progression and after autologous tumor cell vaccines. Clin Cancer Res 9:1656–1665
Müller MR, Tsakou G, Grünebach F et’al (2004) Induction of chronic lymphocytic leukemia (CLL)-specific CD4- and CD8-mediated T-cell responses using RNA-transfected dendritic cells. Blood 103:1763–1769
Author information
Authors and Affiliations
Corresponding author
Additional information
This article forms part of the symposium in writing “T cell therapy in chronic lymphocytic leukemia”, edited by Øystein Bruserud.
Rights and permissions
About this article
Cite this article
Cheson, B.D. Monoclonal antibody therapy of chronic lymphocytic leukemia. Cancer Immunol Immunother 55, 188–196 (2006). https://doi.org/10.1007/s00262-005-0010-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-005-0010-0