Abstract
Purpose
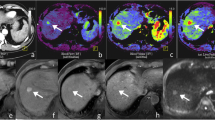
To validate a free-breathing dynamic contrast-enhanced-MRI (DCE-MRI) in hepatocellular carcinoma (HCC) patients using gadoxetic acid, and to determine the relationship between DCE-MRI parameters and histological results.
Methods
Thirty-four HCC patients were included in this prospective study. Free-breathing DCE-MRI data was acquired preoperatively on a 3.0 Tesla scanner. Perfusion parameters (K trans, K ep, V e and the semi-quantitative parameter of initial area under the gadolinium concentration–time curve, iAUC) were calculated and compared with tumor enhancement at contrast-enhanced CT. The relationship between DCE-MRI parameters and Ki67 indices, histological grades and microvascular density (MVD) was determined by correlation analysis. Differences of perfusion parameters between different histopathological groups were compared. Receiver operation characteristic (ROC) analysis of discriminating high-grades (grade III and IV) from low-grades (grade I and II) HCC was performed for perfusion parameters.
Results
Significant relationship was found between DCE-MRI and CT results. The DCE-MRI derived K trans were significantly negatively correlated with Ki-67 indices (rho = − 0.408, P = 0.017) and the histological grades (rho = − 0.444, P = 0.009) of HCC, and K ep and V e were significantly related with tumor MVD (rho = − 0.405, P = 0.017 for K ep; and rho = 0.385, P = 0.024 for V e). K trans, K ep, and iAUC demonstrated moderate diagnostic performance (iAUC = 0.78, 0.77 and 0.80, respectively) for discriminating high-grades from low-grades HCC without significant differences.
Conclusions
The DCE-MRI derived parameters demonstrated weak but significant correlations with tumor proliferation status, histological grades or microvascular density, respectively. This free-breathing DCE-MRI is technically feasible and offers a potential avenue toward non-invasive evaluation of HCC malignancy.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Jonas S, Bechstein WO, Steinmuller T, et al. (2001) Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 33:1080–1086
Perez-Saborido B, De Los Galanes SJ, Meneu-Diaz JC, et al. (2007) Tumor recurrence after liver transplantation for hepatocellular carcinoma: recurrence pathway and prognostic factors. Transplant Proc 39:2304–2307
Zhou L, Rui JA, Wang SB, Chen SG, Qu Q (2015) Clinicopathological predictors of poor survival and recurrence after curative resection in hepatocellular carcinoma without portal vein tumor thrombosis. Pathol Oncol Res 21:131–138
D’Errico A, Grigioni WF, Fiorentino M, et al. (1994) Overexpression of p53 protein and Ki67 proliferative index in hepatocellular carcinoma: an immunohistochemical study on 109 Italian patients. Pathol Int 44:682–687
Hsu HC, Tseng HJ, Lai PL, Lee PH, Peng SY (1993) Expression of p53 gene in 184 unifocal hepatocellular carcinomas: association with tumor growth and invasiveness. Cancer Res 53:4691–4694
Nigro JM, Baker SJ, Preisinger AC, et al. (1989) Mutations in the p53 gene occur in diverse human tumour types. Nature 342:705–708
Stroescu C, Dragnea A, Ivanov B, et al. (2008) Expression of p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in hepatocellular carcinoma. J Gastrointestin Liver Dis 17:411–417
Yamaguchi R, Yano H, Iemura A, et al. (1998) Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology 28:68–77
Zhang W, Kim R, Quintini C, et al. (2015) Prognostic role of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma undergoing liver transplantation. Liver Transpl 21:101–111
Mann CD, Neal CP, Garcea G, et al. (2007) Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer 43:979–992
Murakami K, Kasajima A, Kawagishi N, Ohuchi N, Sasano H (2015) Microvessel density in hepatocellular carcinoma: Prognostic significance and review of the previous published work. Hepatol Res 45:1185–1194
Li Y, Ma X, Zhang J, Liu X, Liu L (2014) Prognostic value of microvessel density in hepatocellular carcinoma patients: a meta-analysis. Int J Biol Markers 29:e279–e287
Tofts PS, Brix G, Buckley DL, et al. (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusible tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Yang X, Knopp MV (2011) Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol 2011:732848
Rao SX, Chen CZ, Liu H, Zeng MS, Qu XD (2013) Three-dimensional whole-liver perfusion magnetic resonance imaging in patients with hepatocellular carcinomas and colorectal hepatic metastases. BMC Gastroenterol 13:53
Huh J, Choi Y, Woo DC, et al. (2016) Feasibility of test-bolus DCE-MRI using CAIPIRINHA-VIBE for the evaluation of pancreatic malignancies. Eur Radiol . doi:10.1007/s00330-016-4209-6
Hao W, Zhao B, Wang G, Wang C, Liu H (2015) Influence of scan duration on the estimation of pharmacokinetic parameters for breast lesions: a study based on CAIPIRINHA-Dixon-TWIST-VIBE technique. Eur Radiol 25:1162–1171
Chen BB, Shih TT (2014) DCE-MRI in hepatocellular carcinoma-clinical and therapeutic image biomarker. World J Gastroenterol 20:3125–3134
Braren R, Curcic J, Remmele S, et al. (2011) Free-breathing quantitative dynamic contrast-enhanced magnetic resonance imaging in a rat liver tumor model using dynamic radial T(1) mapping. Invest Radiol 46:624–631
FritzHansen T, Rostrup E, Larsson HBW, et al. (1996) Measurement of the arterial concentration of Gd-DTPA using MRI: a step toward quantitative perfusion imaging. Magn Reson Med 36:225–231
Wagner M, Doblas S, Daire JL, et al. (2012) Diffusion-weighted MR imaging for the regional characterization of liver tumors. Radiology 264:464–472
Shinriki S, Jono H, Ota K, et al. (2009) Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res 15:5426–5434
Song KD, Choi D, Lee JH, et al. (2014) Evaluation of tumor microvascular response to brivanib by dynamic contrast-enhanced 7-T MRI in an orthotopic xenograft model of hepatocellular carcinoma. AJR Am J Roentgenol 202:W559–W566
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Zwick S, Brix G, Tofts PS, et al. (2010) Simulation-based comparison of two approaches frequently used for dynamic contrast-enhanced MRI. Eur Radiol 20:432–442
Padhani AR, Khan AA (2010) Diffusion-weighted (DW) and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) for monitoring anticancer therapy. Target Oncol 5:39–52
Shen FU, Lu J, Chen L, Wang Z, Chen Y (2016) Diagnostic value of dynamic contrast-enhanced magnetic resonance imaging in rectal cancer and its correlation with tumor differentiation. Mol Clin Oncol 4:500–506
Wegner CS, Gaustad JV, Andersen LM, Simonsen TG, Rofstad EK (2016) Diffusion-weighted and dynamic contrast-enhanced MRI of pancreatic adenocarcinoma xenografts: associations with tumor differentiation and collagen content. J Transl Med 14:161
Kim KA, Park MS, Ji HJ, et al. (2014) Diffusion and perfusion MRI prediction of progression-free survival in patients with hepatocellular carcinoma treated with concurrent chemoradiotherapy. J Magn Reson Imaging 39:286–292
Shin JK, Kim JY (2017) Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: Associations between quantitative MR parameters and Ki-67 proliferation status. J Magn Reson Imaging 45:94–102
Flaherty KT, Rosen MA, Heitjan DF, et al. (2008) Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther 7:496–501
Barnes SL, Whisenant JG, Loveless ME, Yankeelov TE (2012) Practical dynamic contrast enhanced MRI in small animal models of cancer: data acquisition, data analysis, and interpretation. Pharmaceutics 4:442–478
Kim YE, Lim JS, Choi J, et al. (2013) Perfusion parameters of dynamic contrast-enhanced magnetic resonance imaging in patients with rectal cancer: correlation with microvascular density and vascular endothelial growth factor expression. Korean J Radiol 14:878–885
Li L, Wang K, Sun X, et al. (2015) Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit 21:376–382
Jia ZZ, Gu HM, Zhou XJ, et al. (2015) The assessment of immature microvascular density in brain gliomas with dynamic contrast-enhanced magnetic resonance imaging. Eur J Radiol 84:1805–1809
Chen J, Qian T, Zhang H, et al. (2016) Combining dynamic contrast enhanced magnetic resonance imaging and microvessel density to assess the angiogenesis after PEI in a rabbit VX2 liver tumor model. Magn Reson Imaging 34:177–182
Bali MA, Metens T, Denolin V, et al. (2011) Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology 261:456–466
Mayr NA, Hawighorst H, Yuh WT, et al. (1999) MR microcirculation assessment in cervical cancer: correlations with histomorphological tumor markers and clinical outcome. J Magn Reson Imaging 10:267–276
Cheng HL, Wallis C, Shou Z, Farhat WA (2007) Quantifying angiogenesis in VEGF-enhanced tissue-engineered bladder constructs by dynamic contrast-enhanced MRI using contrast agents of different molecular weights. J Magn Reson Imaging 25:137–145
Kim BK, Han KH, Park YN, et al. (2008) Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J Surg Oncol 97:246–252
Oishi K, Itamoto T, Amano H, et al. (2007) Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol 95:311–316
Qian T, Chen M, Gao F, et al. (2014) Diffusion-weighted magnetic resonance imaging to evaluate microvascular density after transarterial embolization ablation in a rabbit VX2 liver tumor model. Magn Reson Imaging 32:1052–1057
An C, Park MS, Jeon HM, et al. (2012) Prediction of the histopathological grade of hepatocellular carcinoma using qualitative diffusion-weighted, dynamic, and hepatobiliary phase MRI. Eur Radiol 22:1701–1708
Chang WC, Chen RC, Chou CT, et al. (2014) Histological grade of hepatocellular carcinoma correlates with arterial enhancement on gadoxetic acid-enhanced and diffusion-weighted MR images. Abdom Imaging 39:1202–1212
Choi YS, Rhee H, Choi JY, et al. (2013) Histological characteristics of small hepatocellular carcinomas showing atypical enhancement patterns on gadoxetic acid-enhanced MR imaging. J Magn Reson Imaging 37:1384–1391
Kogita S, Imai Y, Okada M, et al. (2010) Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol 20:2405–2413
Tahir B, Sandrasegaran K, Ramaswamy R, et al. (2011) Does the hepatocellular phase of gadobenate dimeglumine help to differentiate hepatocellular carcinoma in cirrhotic patients according to histological grade? Clin Radiol 66:845–852
Thompson SM, Wang J, Chandan VS, et al. (2017) MR elastography of hepatocellular carcinoma: Correlation of tumor stiffness with histopathology features-Preliminary findings. Magn Reson Imaging 37:41–45
Matsui O, Kobayashi S, Sanada J, et al. (2011) Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 36:264–272
Park YN, Yang CP, Fernandez GJ, et al. (1998) Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol 22:656–662
Kiessling F, Morgenstern B, Zhang C (2007) Contrast agents and applications to assess tumor angiogenesis in vivo by magnetic resonance imaging. Curr Med Chem 14:77–91
Wu L, Lv P, Zhang H, et al. (2015) Dynamic contrast-enhanced (DCE) MRI assessment of microvascular characteristics in the murine orthotopic pancreatic cancer model. Magn Reson Imaging 33:737–760
Lin YH, Hwang RM, Chen BB, et al. (2014) Vascular and hepatic enhancements at MR imaging: comparison of Gd-EOB-DTPA and Gd-DTPA in the same subjects. Clin Imaging 38:287–291
Narita M, Hatano E, Arizono S, et al. (2009) Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 44:793–798
Kitao A, Matsui O, Yoneda N, et al. (2011) The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol 21:2056–2066
Sourbron S, Sommer WH, Reiser MF, Zech CJ (2012) Combined quantification of liver perfusion and function with dynamic gadoxetic acid-enhanced MR imaging. Radiology 263:874–883
Nilsson H, Nordell A, Vargas R, et al. (2009) Assessment of hepatic extraction fraction and input relative blood flow using dynamic hepatocyte-specific contrast-enhanced MRI. J Magn Reson Imaging 29:1323–1331
Acknowledgement
This study was funded by National Natural Science Foundation of China (Grant Number 81471658).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Jie Chen declares that she has no conflict of interest. Author Chenyang Chen declares that she has no conflict of interest. Author Chunchao Xia declares that he has no conflict of interest. Author Zixing Huang declares that he has no conflict of interest. Author Panli Zuo declares that she has no conflict of interest. Author Alto Stemmer declares that he has no conflict of interest. Author Bin Song declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chen, J., Chen, C., Xia, C. et al. Quantitative free-breathing dynamic contrast-enhanced MRI in hepatocellular carcinoma using gadoxetic acid: correlations with Ki67 proliferation status, histological grades, and microvascular density. Abdom Radiol 43, 1393–1403 (2018). https://doi.org/10.1007/s00261-017-1320-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1320-3