Abstract
Purpose
To assess the performance of the updated Prostate Imaging Reporting and Data System (PI-RADSv2) and the apparent diffusion coefficient (ADC) for predicting confirmatory biopsy results in patients considered for active surveillance of prostate cancer (PCA).
Methods
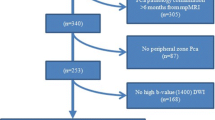
IRB-approved, retrospective study of 371 consecutive men with clinically low-risk PCA (initial biopsy Gleason score ≤6, prostate-specific antigen <10 ng/ml, clinical stage ≤T2a) who underwent 3T-prostate MRI before confirmatory biopsy. Two independent radiologists recorded the PI-RADSv2 scores and measured the corresponding ADC values in each patient. A composite score was generated to assess the performance of combining PI-RADSv2 + ADC.
Results
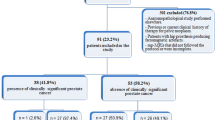
PCA was upgraded on confirmatory biopsy in 107/371 (29%) patients. Inter-reader agreement was substantial (PI-RADSv2: k = 0.73; 95% CI [0.66–0.80]; ADC: r = 0.74; 95% CI [0.69–0.79]). Accuracies, sensitivities, specificities, positive predicted value and negative predicted value of PI-RADSv2 were 85, 89, 83, 68, 95 and 78, 82, 76, 58, 91% for ADC. PI-RADSv2 accuracy was significantly higher than that of ADC for predicting biopsy upgrade (p = 0.014). The combined PI-RADSv2 + ADC composite score did not perform better than PI-RADSv2 alone. Obviating biopsy in patients with PI-RADSv2 score ≤3 would have missed Gleason Score upgrade in 12/232 (5%) of patients.
Conclusion
PI-RADSv2 was superior to ADC measurements for predicting PCA upgrading on confirmatory biopsy.



Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- PCA:
-
Prostate cancer
- T2WI:
-
T2-weighted images
- MRI:
-
Magnetic resonance imaging
- DW:
-
Diffusion-weighted
- DCE:
-
Dynamic contrast-enhanced
- PI-RADS:
-
Prostate imaging reporting and data system
- SD:
-
Standard deviation
References
Adamy A, Yee DS, Matsushita K, et al. (2011) Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol 185:477–482
Wong LM, Alibhai SM, Trottier G, et al. (2014) A negative confirmatory biopsy among men on active surveillance for prostate cancer does not protect them from histologic grade progression. Eur Urol 66:406–413
Wong LM, Ferrara S, Alibhai SM, et al. (2015) Diagnostic prostate biopsy performed in a non-academic center increases the risk of re-classification at confirmatory biopsy for men considering active surveillance for prostate cancer. Prostate Cancer Prostatic Dis 18:69–74
Dall’Era MA, Albertsen PC, Bangma C, et al. (2012) Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 62:976–983
Porten SP, Whitson JM, Cowan JE, et al. (2011) Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol 29:2795–2800
Porten SP, Whitson JM, Cowan JE, et al. (2011) Changes in cancer volume in serial biopsies of men on active surveillance for early stage prostate cancer. J Urol 186:1825–1829
Recabal P, Ehdaie B (2015) The role of MRI in active surveillance for men with localized prostate cancer. Curr Opin Urol 25:504–509
Klotz L (2015) Active surveillance for low-risk prostate cancer. Curr Urol Rep 16:24
Klotz L (2015) Active surveillance for prostate cancer: debate over the application, not the concept. Eur Urol 67:1006–1008
Loeb S, Bruinsma SM, Nicholson J, et al. (2015) Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol 67:619–626
Tosoian JJ, Sundi D, Trock BJ, et al. (2015) Pathologic outcomes in favorable-risk prostate cancer: comparative analysis of men electing active surveillance and immediate surgery. Eur Urol. doi:10.1016/j.eururo.2015.09.032
Bruinsma SM, Bokhorst LP, Roobol MJ, Bangma CH (2015) How often is biopsy necessary in patients with prostate cancer on active surveillance? J Urol. doi:10.1016/j.juro.2015.10.061
Bosco C, Cozzi G, Kinsella J, et al. (2016) Confirmatory biopsy for the assessment of prostate cancer in men considering active surveillance: reference centre experience. Ecancermedicalscience 10:633
Partin AW (2000) Does sextant prostate biopsy provide adequate sampling for early detection of prostate cancer? Curr Urol Rep 1:245
Patel A (2007) Finding a balanced strategy in prostate cancer diagnosis for case detection by prostate needle biopsy at first presentation: “all for more cores and more cores for all” or individualised sampling regimens? Which way forward? Eur Urol 52:313–315
Rodrigues A, Freitas R, Nogueira-Silva P, Jeronimo C, Henrique R (2014) Biopsy sampling and histopathological markers for diagnosis of prostate cancer. Expert Rev Anticancer Ther 14:1323–1336
Vilanova JC, Comet J, Capdevila A, et al. (2001) The value of endorectal MR imaging to predict positive biopsies in clinically intermediate-risk prostate cancer patients. Eur Radiol 11:229–235
Vargas HA, Akin O, Afaq A, et al. (2012) Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol 188:1732–1738
Fascelli M, George AK, Frye T, et al. (2015) The role of MRI in active surveillance for prostate cancer. Curr Urol Rep 16:42
Pessoa RR, Viana PC, Mattedi RL, et al. (2016) Value of 3-Tesla multiparametric magnetic resonance imaging and targeted biopsy for improved risk stratification in patients considered for active surveillance. BJU Int. doi:10.1111/bju.13624
Vargas HA, Akin O, Shukla-Dave A, et al. (2012) Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology 265:478–487
Bratan F, Melodelima C, Souchon R, et al. (2015) How accurate is multiparametric MR imaging in evaluation of prostate cancer volume? Radiology 275:144–154
Donati OF, Jung SI, Vargas HA, et al. (2013) Multiparametric prostate MR imaging with T2-weighted, diffusion-weighted, and dynamic contrast-enhanced sequences: are all pulse sequences necessary to detect locally recurrent prostate cancer after radiation therapy? Radiology 268:440–450
Hoeks CM, Barentsz JO, Hambrock T, et al. (2011) Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 261:46–66
Kim JY, Kim SH, Kim YH, et al. (2014) Low-risk prostate cancer: the accuracy of multiparametric MR imaging for detection. Radiology 271:435–444
Peng Y, Jiang Y, Antic T, et al. (2014) Validation of quantitative analysis of multiparametric prostate MR images for prostate cancer detection and aggressiveness assessment: a cross-imager study. Radiology 271:461–471
Kayat Bittencourt L, Litjens G, Hulsbergen-van de Kaa CA, et al. (2015) Prostate cancer: the european society of urogenital radiology prostate imaging reporting and data system criteria for predicting extraprostatic extension by using 3-t multiparametric MR imaging. Radiology. doi:10.1148/radiol.15141412:141412
Vargas HA, Hotker AM, Goldman DA, et al. (2015) Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. doi:10.1007/s00330-015-4015-6
Weinreb JC, Barentsz JO, Choyke PL, et al. (2016) PI-RADS prostate imaging—reporting and data system: 2015, Version 2. Eur Urol 69:16–40
Ross AE, Loeb S, Landis P, et al. (2010) Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol 28:2810–2816
Guo R, Cai L, Fan Y, et al. (2015) Magnetic resonance imaging on disease reclassification among active surveillance candidates with low-risk prostate cancer: a diagnostic meta-analysis. Prostate Cancer Prostatic Dis 18:221–228
van As NJ, de Souza NM, Riches SF, et al. (2009) A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol 56:981–987
Zhao C, Gao G, Fang D, et al. (2016) The efficiency of multiparametric magnetic resonance imaging (mpMRI) using PI-RADS Version 2 in the diagnosis of clinically significant prostate cancer. Clin Imaging 40:885–888
Vargas HA, Hotker AM, Goldman DA, et al. (2016) Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 26:1606–1612
Liddell H, Jyoti R, Haxhimolla HZ (2015) mp-MRI prostate characterised PIRADS 3 lesions are associated with a low risk of clinically significant prostate cancer—a retrospective review of 92 biopsied PIRADS 3 lesions. Curr Urol 8:96–100
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partially funded by NIH grant P30 (CA008748) (Evis Sala, Hedvik Hricak, Alberto Vargas).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
The institutional review board approved this retrospective study and waived the informed consent requirement.
Rights and permissions
About this article
Cite this article
Nougaret, S., Robertson, N., Golia Pernicka, J. et al. The performance of PI-RADSv2 and quantitative apparent diffusion coefficient for predicting confirmatory prostate biopsy findings in patients considered for active surveillance of prostate cancer. Abdom Radiol 42, 1968–1974 (2017). https://doi.org/10.1007/s00261-017-1086-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1086-7